To import cosmetics into India, an importer must obtain approvals from multiple regulatory authorities, not just CDSCO. Many businesses assume that cosmetic imports are governed by a single license. However, in reality, cosmetic import is a multi-compliance process regulated under the Drugs and Cosmetics Act, 1940, Cosmetic Rules, 2020, Legal Metrology laws, and environmental regulations.
Table of Contents
ToggleWhat approvals are required to import cosmetics into India?
Before any cosmetic shipment can legally enter India, the required approvals to import cosmetics into India are:
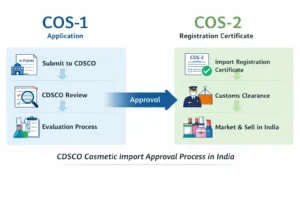
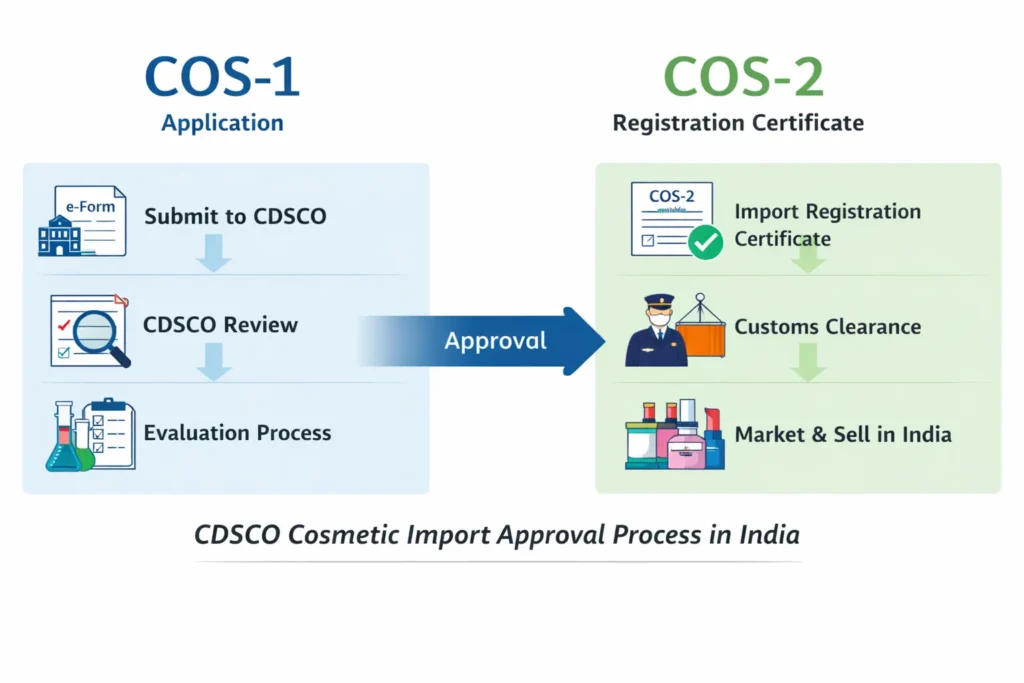
CDSCO Cosmetic Import Registration Certificate (COS-1 / COS-2)
Importer Registration under Legal Metrology (LMPC Rules, 2011)
EPR Authorization from CPCB (In case the product is packed in plastic packaging)
Importers must first understand the CDSCO Cosmetic License for Importer (COS-1 & COS-2), which forms the foundation of cosmetic import compliance in India. This registration, issued by the CDSCO, authorizes the legal import, storage, and sale of cosmetic products in the Indian market. Without this approval, cosmetic consignments cannot clear customs or be distributed in India, making it a critical requirement before initiating any import-related activity.
Therefore, understanding how these approvals work together is essential for smooth customs clearance and long-term compliance. This guide explains the step-by-step CDSCO process for importing cosmetics into India, focusing on execution and readiness rather than regulatory theory.
Why Importing Cosmetics into India Requires Multiple Regulatory Approvals
Cosmetic imports in India are regulated by CDSCO, but approval from other authorities is mandatory because cosmetics directly impact consumer safety, labeling transparency, and environmental responsibility. CDSCO evaluates product safety and ingredient compliance, Legal Metrology ensures correct packaging declarations, and CPCB regulates plastic waste through EPR authorization.
Together, these approvals form a comprehensive regulatory framework that protects consumers while holding importers accountable for compliance across the product lifecycle.
Step-by-Step Process to Import Cosmetics into India
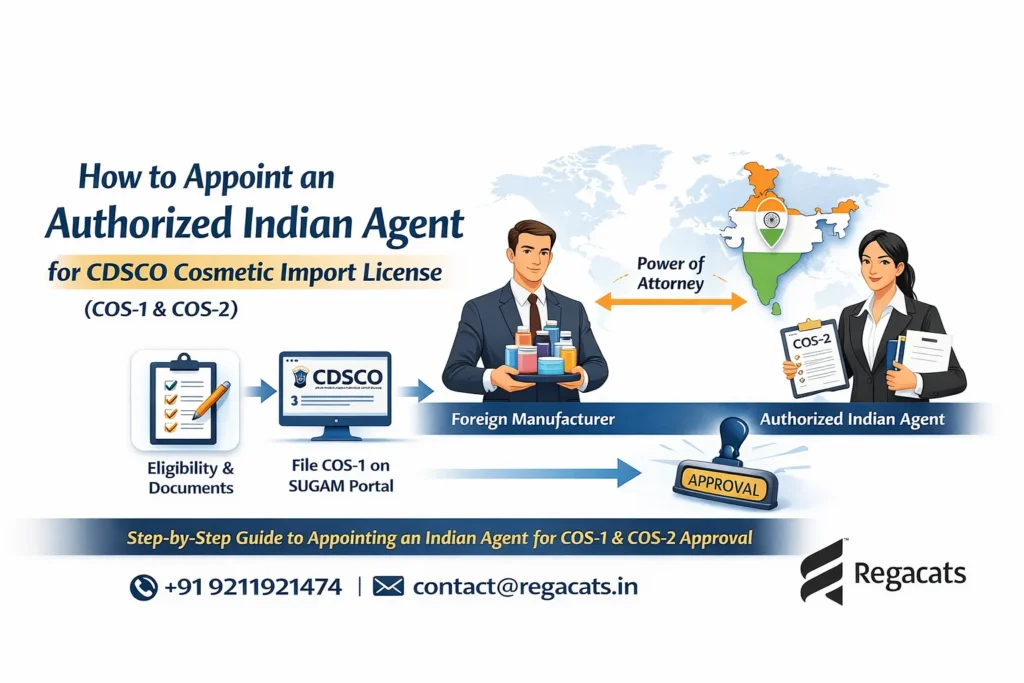
Step 1: Identification of Foreign Manufacturer
First, the importer must identify a foreign cosmetic manufacturer that complies with both home-country regulations and Indian cosmetic standards. Importantly, the manufacturer must legally manufacture and sell cosmetics in its country of origin.
At this stage, importers must verify that the foreign manufacturer:
Holds a valid cosmetic manufacturing license
Is legally allowed to sell the product in the country of origin
Has a consistent factory address across all documents
Is willing to authorize an Indian importer or agent
Additionally, the manufacturer must issue a Power of Attorney authorizing the Indian importer to apply for cosmetic import approvals in India. This document establishes the legal relationship between the manufacturer and the importer and becomes a critical part of the CDSCO application.
Step 2: Prepare Documents for Cosmetic Import Registration
Once the foreign manufacturer is finalized, the next step is document preparation, which plays a decisive role in approval timelines. Most regulatory delays arise from documentation errors.
At this stage, importers must prepare and validate:
Power of Attorney from the manufacturer
Free Sale Certificate issued by the country of origin
Manufacturing license of the cosmetic facility
Complete ingredient list with composition percentages
Product specifications and safety data
Cosmetic label artwork compliant with Indian rules
Importantly, all documents must be internally consistent. Product names, variant details, manufacturer address, and importer details must match exactly across every submission. Even small discrepancies often result in CDSCO queries.
In parallel, documentation for Legal Metrology (LMPC) registration and EPR authorization should also be prepared to avoid approval gaps later.
Step 3: Obtain CDSCO, LMPC, and EPR Approvals
After document preparation, applications are submitted to the relevant authorities.
1. CDSCO Cosmetic Import Registration:
The cosmetic import application (Cos-1) is submitted online through the CDSCO SUGAM portal. CDSCO evaluates product ingredients, safety data, labeling compliance, and manufacturer credentials. Upon satisfaction, CDSCO issues the COS-2 registration certificate, which legally permits cosmetic imports into India.
2. Legal Metrology (LMPC) Importer Registration:
Separately, importers must obtain registration under Rule 27 of the Legal Metrology (Packaged Commodity) Rules, 2011. This approval ensures that imported cosmetics comply with Indian packaging and quantity declaration standards.
3. EPR Authorization from CPCB:
In addition, cosmetic importers must secure Extended Producer Responsibility (EPR) authorization from the Central Pollution Control Board (CPCB). This approval covers compliance for plastic packaging waste generated from imported cosmetic products.
Regulatory Approvals Required to Import Cosmetics into India
| Authority | Approval Required | Purpose |
|---|---|---|
| CDSCO | Cosmetic Import Registration (COS-1 & COS-2) | Product safety and compliance |
| Legal Metrology | LMPC Importer Registration | Packaging and labeling accuracy |
| CPCB | EPR Authorization | Plastic waste management |
Step 4: Customs Clearance and Import of Cosmetics into India
Once all regulatory approvals are obtained, the final operational step is customs clearance of cosmetic consignments.
At the port of entry, customs officials verify:
CDSCO COS-2 certificate
LMPC importer registration
EPR authorization details
Invoice, packing list, and shipping documents
Product labels and batch information
If any approval is missing or inconsistent, customs authorities may detain, re-export, or order destruction of the shipment. Therefore, importers should conduct a pre-clearance compliance check before dispatching goods.
After successful customs clearance, cosmetics can be legally stored, distributed, and sold across India through retail stores, distributors, or e-commerce platforms.
Common Mistakes to Avoid While Importing Cosmetics into India
Although the process appears straightforward, many importers face delays due to avoidable mistakes. Therefore, understanding common compliance errors helps reduce regulatory risks.
Common mistakes include:
Ingredient mismatches with Indian standards
Incorrect or incomplete labeling
Poorly drafted Power of Attorney
Inconsistent manufacturer details across documents
Addressing these issues early significantly improves approval success rates.
How This Step-by-Step Approach Reduces Import Risk
Following a structured import process helps businesses:
Avoid regulatory penalties
Prevent shipment detention
Reduce approval delays
Maintain long-term compliance
Ultimately, proper execution protects both business investment and brand reputation in the Indian market.
How Regacats Solutions Supports Cosmetic Importers
Since cosmetic import involves multiple authorities and strict documentation checks, many importers prefer working with experienced regulatory professionals. Regacats Solutions provides CDSCO cosmetic import license consulting services in India, assisting importers with COS-1 and COS-2 registrations, documentation review, query handling, and end-to-end approval support. Professional guidance helps reduce approval timelines and ensures long-term compliance with Indian cosmetic regulations.
The Regacats team also supports businesses with:
Foreign manufacturer identification
Documentation and label compliance review
LMPC importer registration
EPR authorization from CPCB
This integrated approach ensures approval certainty, speed, and regulatory confidence.
Conclusion
In conclusion, importing cosmetics into India requires careful coordination across CDSCO, Legal Metrology, and CPCB. By identifying a compliant foreign manufacturer, preparing accurate documentation, securing all required approvals, and planning customs clearance in advance, importers can enter the Indian cosmetic market smoothly and legally.
This step-by-step CDSCO process serves as a practical execution guide, while the pillar page provides the complete regulatory framework for cosmetic import compliance in India.