Page Contents
ToggleCDSCO Cosmetic Import Registration or License in India
The CDSCO mandates the Cosmetic Import License (also known as Cosmetic Import Registration) as a mandatory regulatory approval for importing cosmetic products into India. Under the Drugs and Cosmetics Act, 1940 and Cosmetics Rules, 2020, CDSCO grants this approval to authorized Indian entities, enabling them to legally import, sell, and distribute cosmetic products in India.
Any foreign cosmetic manufacturer, Exporter, Global brand or Indian importer planning to sell skincare, haircare, makeup, personal care products or other cosmetic products in India must obtain this license or registration before importing goods into India. The terms cosmetic import license and cosmetic import registration are often used interchangeably in India.
Therefore, understanding the CDSCO Cosmetic Import License – specifically COS-1 and COS-2 is critical for importers who want smooth customs clearance, uninterrupted sales, and long-term regulatory compliance.
This guide by Regacats Solutions explains what the CDSCO Cosmetic Import License or Cosmetic Import Registration is, who needs it, how to apply, documents required, fees, approval timelines, labeling rules, and common compliance mistakes, based on practical regulatory experience.
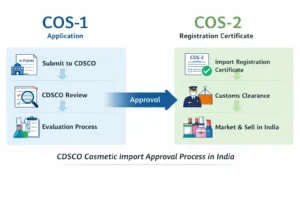
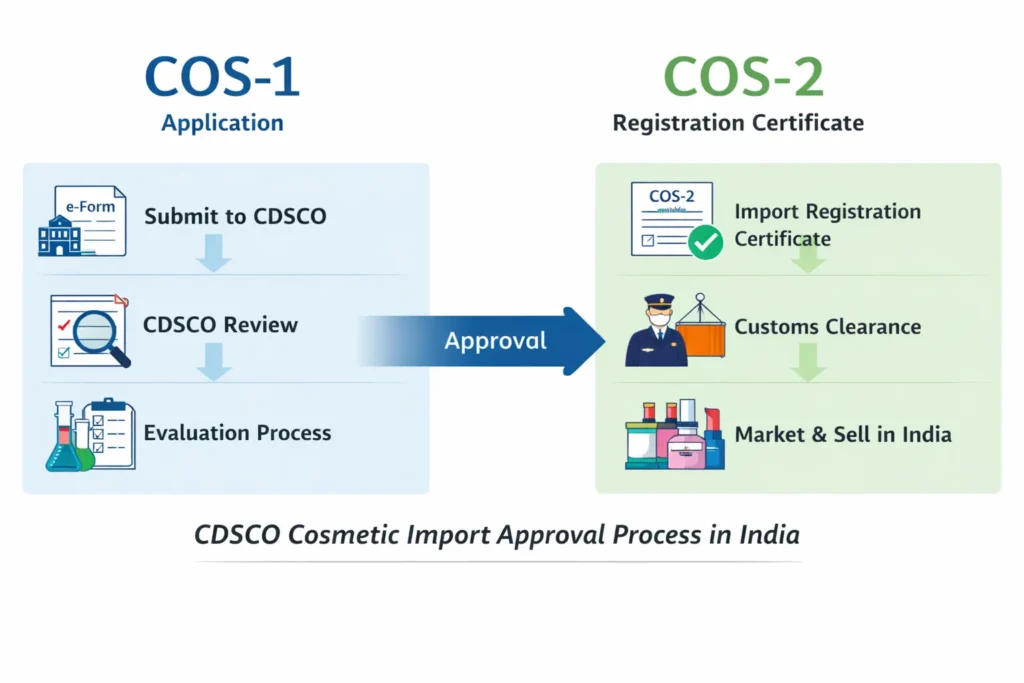
What Is the CDSCO Cosmetic Import License or Cosmetic Import Registration (Cos-1 & Cos-2)?
CDSCO cosmetic import license or registration (Cos-2) is an approval granted by CDSCO allowing the import of cosmetic products into India for sale, distribution, or marketing. It authorizes an Indian entity to legally import cosmetic products into India for sale, distribution, or marketing.
Importers must secure this registration before customs clearance. Indian law does not permit post-import registration or retrospective approvals. The COS-2 license confirms that imported cosmetic products:
Use ingredients permitted under Indian cosmetic & BIS standards
Meet safety and quality requirements
Comply with Indian labeling norms
Protect public health and consumer safety
What Are Cos-1 and Cos-2?
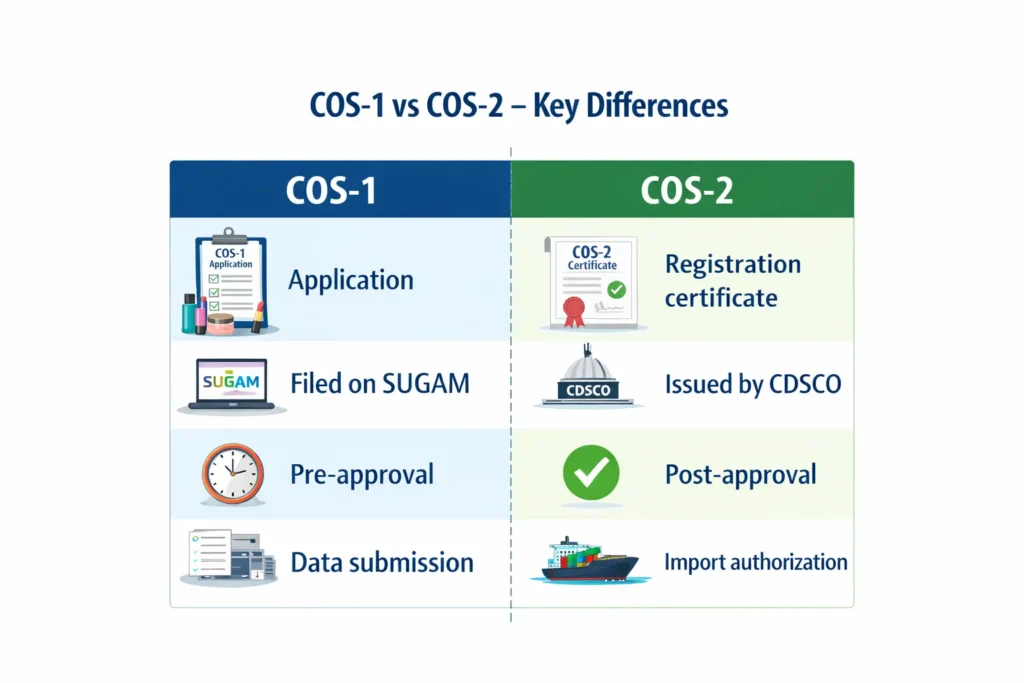
COS-1 is the online application submitted to CDSCO for cosmetic import registration, while After reviewing the COS-1 application submitted on the SUGAM portal, CDSCO issues the COS-2 registration certificate on Sugam Portal. These terms are widely used across the industry to describe the registration stages.
What is Cos-1 (Cosmetic Import Registration Application)
The Cos-1 is a primary application filed on CDSCO SUGAM portal to obtain cosmetic import registration. It includes importer, foreign manufacturer, foreign manufacturing site, and product details.
Cos-1 typically covers:
Foreign Manufacturer and site details
Product details
Importer details
What is Cos-2 (Cosmetic Import Registration Certificate or Cosmetic Import License)
The COS-2 certificate allows importers to import only those cosmetic products that they included in the approved COS-1 application during Cos-1 application submission.
Once CDSCO issues the COS-2 certificate, importers may import only the approved cosmetic products, variants, and manufacturing sites that were approved under the corresponding COS-1 application.
Who Needs a CDSCO Cosmetic Import License or Registration in India?
Any entity involved in importing cosmetics into India must comply with cosmetic import registration requirements in India. This includes:
Indian cosmetic importers or distributors
Authorized Indian agents appointed by foreign manufacturers or exporter
Indian subsidiaries of overseas cosmetic brands
Indian Manufacturers
Applicants must have:
A legally registered Indian entity
Import Export Code (IEC)
GST registration
Authorization from the foreign manufacturer
Foreign manufacturers cannot apply directly without appointing an Indian authorized agent.
When is the CDSCO Cosmetic Import License or Registration is Mandatory?
CDSCO cosmetic import registration is mandatory before:
Importing cosmetic products into India
Clearing goods through Indian customs
Selling cosmetics online or offline on own brand name
Exporting cosmetics into India
Indian customs authorities treat any cosmetic consignment imported without prior CDSCO approval as non-compliant and may detain, reject, or confiscate it.
CDSCO Cosmetic Import License or Registration Approval Process Overview
The CDSCO cosmetic import registration process involves CDSCO assessing both the manufacturer and the cosmetic products.
At a high level, the CDSCO cosmetic import registration process includes:
1. Appointment of an authorized Indian agent
2. Preparation of regulatory documentation
3. Online submission through CDSCO SUGAM portal
4. Payment of government fees
5. Regulatory review and query resolution
6. CDSCO issues the COS-2 registration certificate
In practice, applications with accurate documentation and compliant labels move faster.
Step-by-Step Process to Obtain CDSCO Cosmetic Import Registration or License
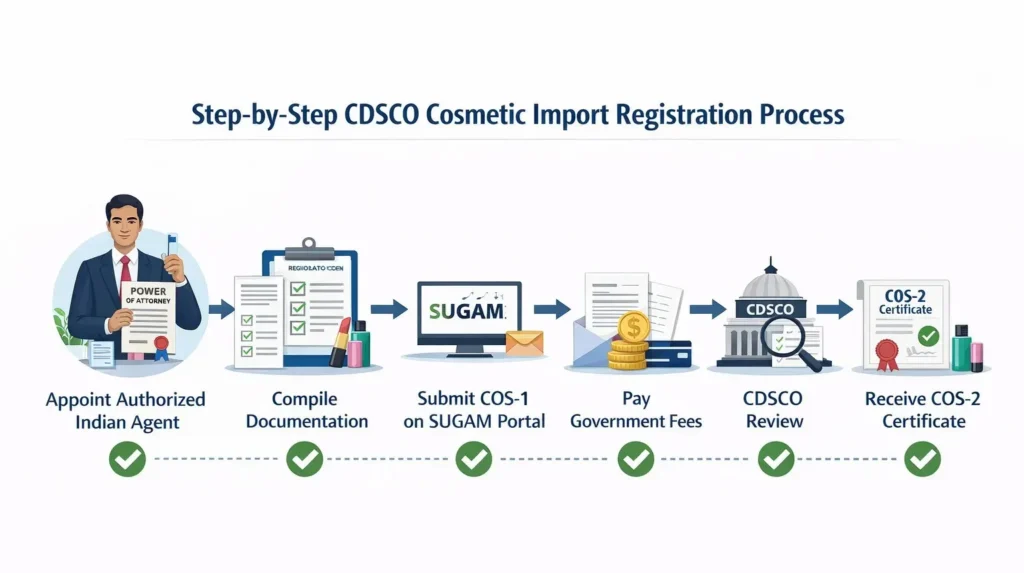
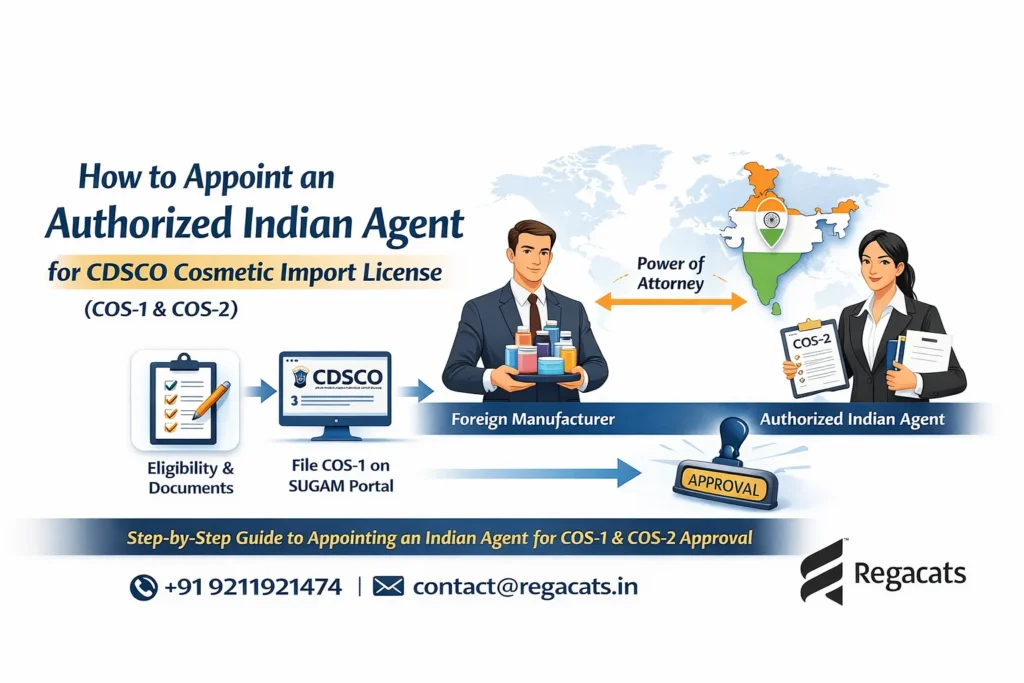
1. Appointment of Authorized Indian Agent
Initially, A foreign manufacturer issues a Power of Attorney authorizing an Indian entity to apply for the CDSCO Cosmetic License for Importer.
2. Preparation of Regulatory Documents
At this stage, Applicants compile all product and manufacturer documents in line with CDSCO requirements.
3. Online Submission on SUGAM Portal
Next, Applicants file the COS-1 application electronically through the CDSCO SUGAM portal.
4. Payment of Government Fees
Thereafter, Applicants pay the applicable government fees online.
5. Regulatory Review by CDSCO
During the review process, CDSCO may raise queries related to ingredients, labeling, or safety data.
6. Grant of COS-2 Registration
Finally, CDSCO issues COS-2 certificate upon satisfactory review.
After approval, the issued COS-1 & COS-2 registration enables the importer to legally clear cosmetic consignments through Indian customs and distribute products across India, subject to ongoing compliance with labeling and post-market regulations.
Detailed Guides on Cosmetic Import Registration in India
While this guide provides a complete overview of the CDSCO Cosmetic Import License (COS-1 & COS-2), importers often need deeper clarity on specific compliance areas. To support practical execution and avoid common regulatory mistakes, refer to the following detailed guides:
What are the Documents Required for Cosmetic Import Registration or License (Overview)
Importers must submit accurate, complete, and compliant documents for cosmetic import registration. Accurate documentation significantly impacts approval timelines.
CDSCO evaluates both manufacturer-level documents and product-specific documents during cosmetic import registration review.
Importers must submit:
Power of Attorney from manufacturer
Free Sale Certificate from the country of origin
Manufacturing license
Complete ingredient list with composition
Product specifications and safety data
Label artwork compliant with Indian rules
Covering letter and declarations
What are the Cosmetic Labeling Requirements for Imported Cosmetics
Compliance with Indian cosmetic labeling requirements in India is mandatory before import. Every cosmetic label must include:
Product name
Manufacturer’s name and address
Importer’s name and address
Batch number
Manufacturing and expiry date
Net quantity
“Imported by” declaration
Consumer care details
Precautions to use product, if any
MRP, USP
Importantly, Importers must complete label compliance before import, as Indian law does not permit post-import relabeling of cosmetics.
CDSCO Fees for Cosmetic Import License
The CDSCO cosmetic import registration fees depend upon:
Number of product categories
Number of manufacturing sites
Number of variants
CDSCO charges government fees of USD 1,000 per category, USD 500 per manufacturing site, and USD 50 per variant.
Importers pay these government fees only once per approved category, site, or variant, not per shipment.
Since fees are non-refundable, accurate classification during filing is essential.
Timeline for CDSCO Cosmetic Import Registration (Cos-1, Cos-2) Approval
A realistic approval timeline is:
2 to 3 months for standard applications
Additional time if CDSCO raises technical or documentation queries
Approval timelines may vary based on product formulation, labeling accuracy, and responsiveness to CDSCO queries.
Applications with compliant ingredients, labels, and consistent documentation typically receive faster approvals.
With expert handling, Regacats Solutions typically completes approvals in around 40 days.
Common Mistakes in Cosmetic Import Registration or License
Many applications face delays due to common mistakes in cosmetic import registration or license. Avoidable errors cause most delays and rejections in cosmetic import registration applications.
Ingredient non-compliance with Indian standards
Incorrect or incomplete labeling
Poorly drafted Power of Attorney
Mismatch in manufacturer details
Incorrect product categorization
How Regacats Solutions Helps with CDSCO Cosmetic Import License or Registration
Regacats Solutions provides end-to-end CDSCO cosmetic import license or registration support, including documentation, filing, query handling, and post-approval compliance.
Regacats Solutions supports cosmetic importers with:
Eligibility assessment
Documentation and labeling compliance
CDSCO SUGAM portal filing
Regulatory query handling
Ongoing compliance advisory
Our approach focuses on accuracy, approval certainty, and sustainable compliance.
Regacats Solutions acts as an authorized indian agent and regulatory compliance partner for cosmetic importers, foreign manufacturer ensuring CDSCO alignment from application to post-approval stages.
Frequently Asked Questions on CDSCO Cosmetic Import License
About Regacats Solutions
Regacats Solutions is an India-based regulatory consulting firm specializing in CDSCO cosmetic import licensing, medical device import registration, and FSSAI regulatory compliance. With extensive experience supporting Indian importers and global cosmetic brands, we assist with COS-1 and COS-2 registration, labeling compliance, Legal Metrology requirements, and post-approval regulatory obligations. Our team focuses on India-specific compliance frameworks to ensure faster approvals, accurate documentation, and long-term regulatory compliance.
Conclusion
CDSCO mandates the Cosmetic Import License (COS-1 & COS-2) as a regulatory approval for importing cosmetic products into India. With compliant documentation, correct labeling, and expert regulatory support, importers can enter the Indian market confidently and scale responsibly. Contact us today to get Cos-2 approval in around 40 days.