Cosmetic Import Registration: CDSCO COS-1 vs COS-2 Explained
Understanding the difference between COS-1 and COS-2 is critical for businesses planning to import cosmetic products into India. Although many importers casually refer to this approval as a cosmetic import license, CDSCO actually follows a registration-based approval system under the Cosmetics Rules, 2020.
Therefore, knowing what COS-1 and COS-2 mean, when each is required, and how they work together helps importers avoid application errors, regulatory delays, and unnecessary costs. This guide explains the CDSCO cosmetic import approval process clearly and practically, without regulatory jargon.
Table of Contents
ToggleWhat is CDSCO Cosmetic Import Registration Approval?
Before importing cosmetics into India, every importer must obtain approval from the Central Drugs Standard Control Organization (CDSCO). This approval ensures that imported cosmetic products comply with Indian safety, labeling, and quality regulations.
However, CDSCO does not issue a single document called a “cosmetic import license.”
Instead, the approval process involves two separate forms:
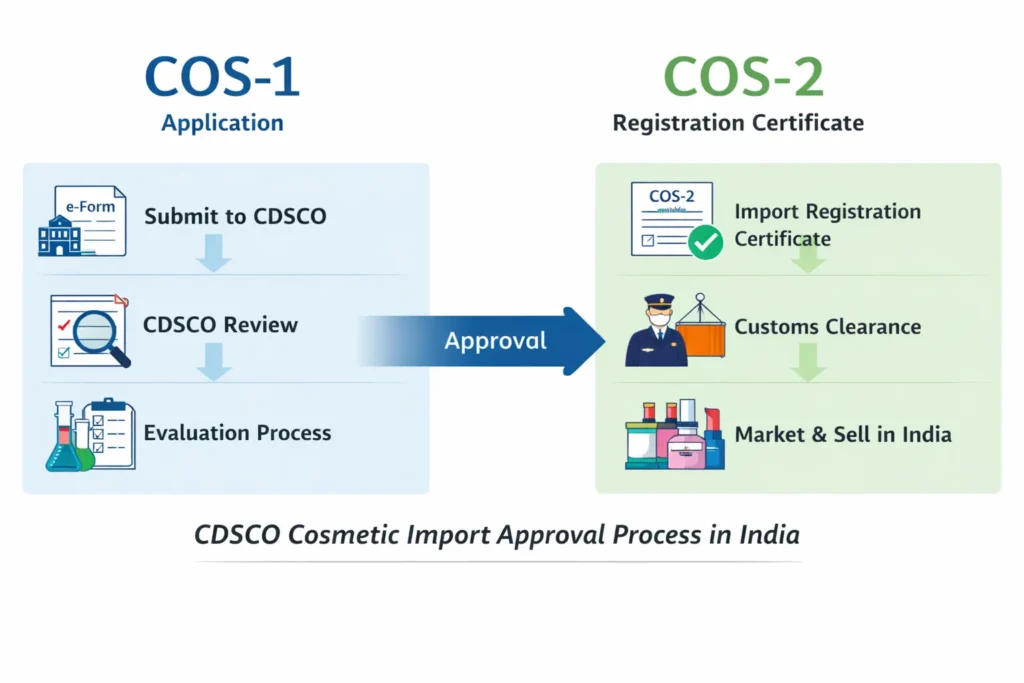
COS-1 – the application form
COS-2 – the import registration certificate issued after approval
Understanding this distinction is essential for smooth compliance.
What is COS-1 in Cosmetic Import Registration?
COS-1 is the application form submitted to CDSCO for registering cosmetic products intended for import into India.
Key points about COS-1:
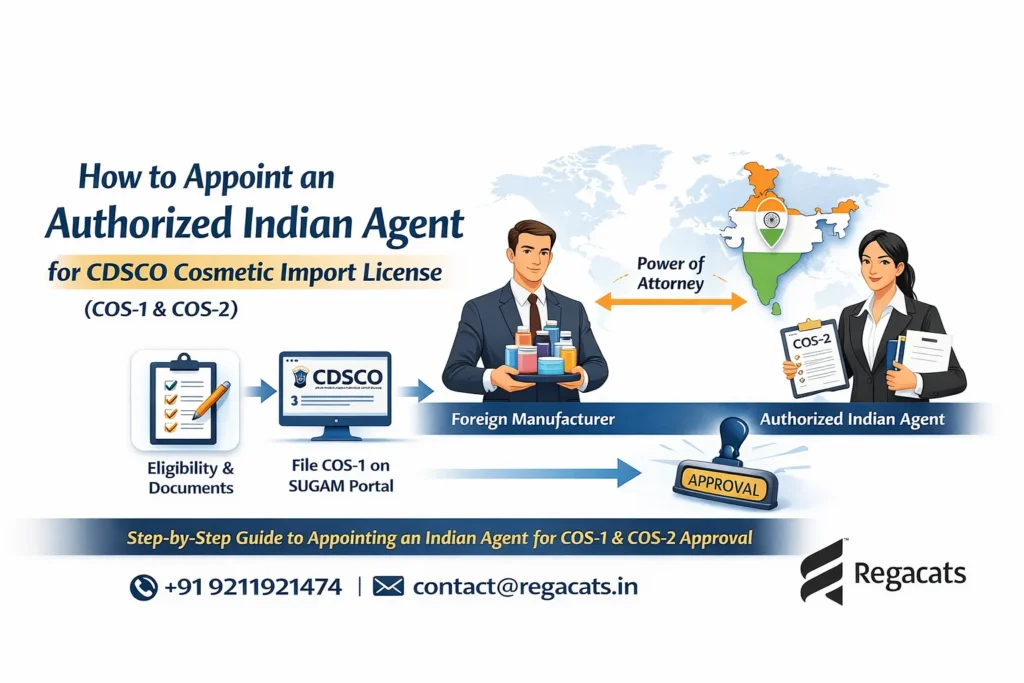
Filed online through the CDSCO SUGAM portal
Submitted by an authorized Indian importer, agent, or subsidiary
Covers details of:
Manufacturer
Manufacturing site
Product category
Variants
Ingredients and labeling
Importers must submit COS-1 before any cosmetic shipment enters India. Without a valid COS-1 application, CDSCO will not evaluate the products.
For a complete understanding of eligibility, documentation, and the overall approval framework, refer to our detailed pillar guide:
Cosmetic Import License in India: CDSCO COS-1 & COS-2 Guide
What is COS-2 in Cosmetic Import Registration?
COS-2 is the Cosmetic Import Registration Certificate issued by CDSCO after successful approval of the COS-1 application.
Key points about COS-2:
Acts as legal permission to import cosmetics into India
Issued only after CDSCO verifies product safety and compliance
Valid for three years
Required at the time of customs clearance
In simple terms, COS-1 starts the process, while COS-2 completes it.
COS-1 vs COS-2: Key Differences Explained
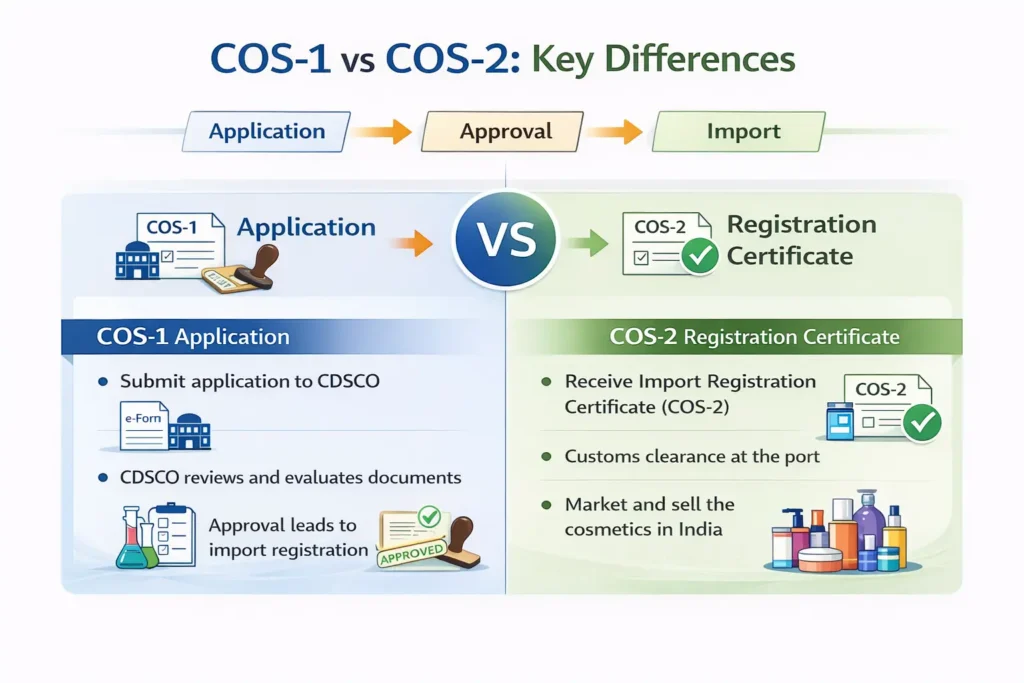
| Aspect | COS-1 | COS-2 |
|---|---|---|
| Purpose | Application for import registration | Import registration certificate |
| Issued by | Applicant submits to CDSCO | CDSCO issues after approval |
| Timing | Before review | After approval |
| Legal authority | No | Yes |
| Used for | Evaluation | Customs clearance & sale |
As a result, importers must treat COS-1 and COS-2 as two connected but distinct stages, not interchangeable documents.
When are COS-1 and COS-2 Required in Cosmetic Import Registration?
COS-1 and COS-2 are required before importing or selling any cosmetic product in India, regardless of whether the products are sold online or offline.
They apply to:
Skincare products
Haircare products
Makeup and personal care items
Imported finished cosmetic formulations
Moreover, foreign manufacturers cannot apply directly. Instead, they must appoint an authorized Indian agent to file COS-1 and hold COS-2.
Common Mistakes Importers Make with COS-1 and COS-2
Although the process appears straightforward, many applications face delays due to avoidable mistakes.
Common errors include:
Treating COS-1 as the final approval
Incorrect classification of cosmetic categories
Mismatch between manufacturer documents and application details
Non-compliant labeling artwork
Inaccurate ingredient declarations
Because CDSCO fees are non-refundable, these mistakes often lead to financial loss and extended timelines.
How CDSCO Reviews COS-1 Applications
Once submitted, CDSCO evaluates:
Ingredient safety as per Indian standards
Compliance with the Cosmetics Rules, 2020
Labeling accuracy
Manufacturer credentials
If CDSCO raises queries, the importer must respond within the specified timeframe. Delayed or incomplete responses often slow down approval.
How Regacats Solutions Supports COS-1 & COS-2 Approvals
Navigating COS-1 and COS-2 requirements requires technical accuracy and regulatory experience. Regacats Solutions provides end-to-end support for cosmetic import approvals, helping importers avoid common pitfalls.
Our services include:
COS-1 application preparation and review
Accurate category and variant classification
Label and ingredient compliance verification
CDSCO SUGAM portal filing
Regulatory query handling until COS-2 issuance
If you need professional assistance with approvals, timelines, and compliance, explore our CDSCO Cosmetic Import License (COS-1, COS-2) Consultant in India services.
About Regacats Solutions
Regacats Solutions is an India-based regulatory consulting firm specializing in CDSCO and statutory compliance for regulated industries. With extensive experience in cosmetic import licensing, medical device registration, and FSSAI regulatory approvals, we support Indian importers and international manufacturers across the entire compliance lifecycle.
Our expertise includes CDSCO cosmetic import registration (COS-1 & COS-2), Legal Metrology compliance, EPR authorization, and post-approval regulatory management. By focusing on India-specific regulatory frameworks, Regacats Solutions helps businesses achieve faster approvals, accurate documentation, and long-term compliance.
Content Reviewed by
Regulatory Experts at Regacats Solutions
Specialists in CDSCO cosmetic import licensing, Legal Metrology compliance, EPR authorization, medical device import registration, and FSSAI regulatory consulting in India.