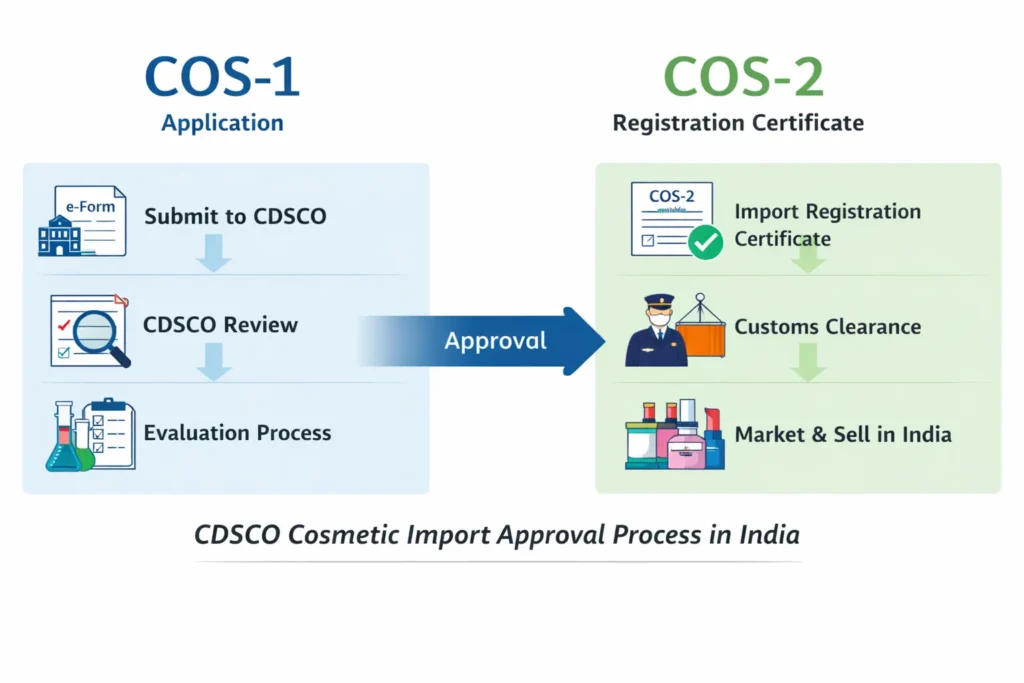
Cosmetic Import License Documents (COS-2) – CDSCO Checklist
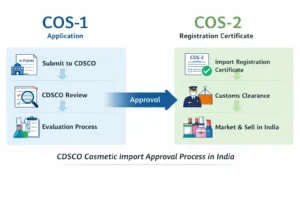
Importing cosmetic products into India requires strict regulatory compliance, and preparing the correct cosmetic import license documents is one of the most critical steps in the CDSCO approval process. Under the Drugs and Cosmetics Act, 1940 and the Cosmetic Rules, 2020, importers must submit accurate manufacturer, importer, and product documentation to obtain a COS-2 license without delays.
However, in practice, most application delays occur not because of product quality, but due to documentation gaps or inconsistencies. Therefore, understanding the complete checklist of cosmetic import license documents is essential for businesses planning to enter the Indian cosmetic market.
This guide explains the documents required for cosmetic import license, based on real CDSCO filing experience, while also highlighting common mistakes that lead to regulatory queries.
This topic forms part of the overall Cosmetic Import License in India: CDSCO COS-1 & COS-2 Guide regulatory framework.
Table of Contents
ToggleWhy Cosmetic Import License Documents Matter for CDSCO Approval
CDSCO evaluates cosmetic import applications primarily on documentation accuracy. Unlike other commercial registrations, CDSCO focuses heavily on regulatory consistency, traceability, and legal authorization.
Moreover, even small discrepancies—such as mismatched manufacturer names, incorrect ingredient nomenclature, or incomplete declarations—can result in formal CDSCO queries. As a result, approval timelines often extend unnecessarily.
Therefore, preparing compliant cosmetic import license documents at the initial stage significantly improves approval certainty and reduces processing time.
Many importers ask whether CDSCO approval is mandatory for cosmetic imports in India, especially when importing products for the first time. This requirement is explained in detail in our dedicated FAQ, which clarifies when CDSCO approval is compulsory and how it impacts cosmetic import compliance.
Cosmetic Import License Documents Required from the Foreign Manufacturer
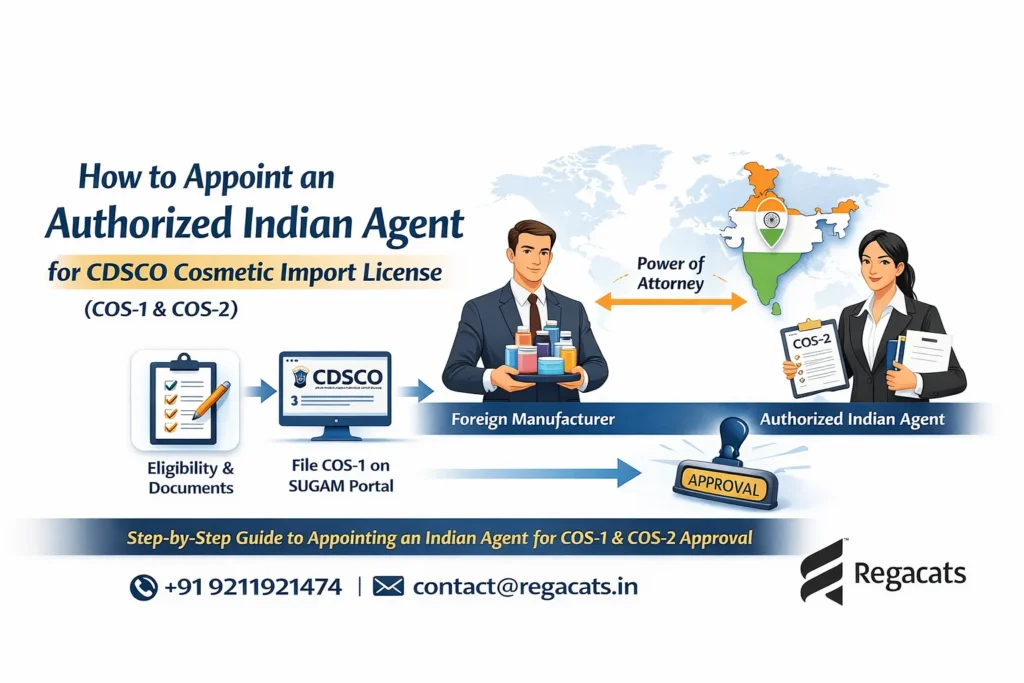
To begin with, the foreign cosmetic manufacturer must provide several mandatory documents to submit on Sugam Portal.
Power of Attorney (PoA)
The Power of Attorney authorizes the Indian importer or agent to act on behalf of the manufacturer for CDSCO registration. Importantly, CDSCO requires the PoA to be:
Issued on the manufacturer’s official letterhead
Notarized and apostilled or legalized
Valid for the license duration
Since CDSCO carefully reviews PoA formatting and execution, any error at this stage often leads to rejection.
Free Sale Certificate (FSC)
Next, the manufacturer must submit a Free Sale Certificate confirming that the cosmetic products are legally sold in the country of origin. Typically, regulatory authorities, export councils, or chambers of commerce issue this document.
Additionally, product names and variants mentioned in the FSC must exactly match the COS-1 application, otherwise CDSCO may raise objections.
Manufacturing License or GMP Certificate
Furthermore, CDSCO requires proof that the manufacturing site operates in compliance with local regulations. Acceptable documents include:
Cosmetic manufacturing license
GMP certificate (ISO 22716 preferred)
Site registration certificate
Each manufacturing location included in the application must be supported individually.
Cosmetic Import License Documents Required from the Indian Importer
In parallel, the Indian applicant must demonstrate legal eligibility to import and distribute cosmetics in India.
Importer Registration Documents
The importer must submit
Import Export Code (IEC)
GST registration
Authorized signatory details
Since CDSCO accepts applications only from Indian-registered entities, these documents remain mandatory.
Cover Letter to CDSCO
Additionally, a formal covering letter must accompany the application. This letter should clearly outline:
Purpose of the application
Product categories and variants
Manufacturer details
List of enclosed cosmetic import license documents
Because CDSCO reviewers rely on this letter as a reference point, clear structuring improves readability and review efficiency.
Product-Specific Documents
After confirming manufacturer and importer eligibility, CDSCO examines product-level compliance documents.
Ingredient List
Every cosmetic product must include a complete ingredient list using INCI nomenclature. Moreover, importers must ensure compliance with:
Prohibited ingredients list
Restricted ingredient concentration limits
Approved preservatives and colorants
Incorrect ingredient naming remains one of the most common reasons for CDSCO objections.
Product Specifications and Safety Data
In addition, importers must submit:
Product specifications
Testing methods
Safety justification
These documents demonstrate that the cosmetic product is safe for consumer use under Indian conditions.
Artwork
Equally important, label artwork must comply with Cosmetic Rules, 2020 and Legal Metrology requirements. Mandatory declarations include:
Product name and category
Manufacturer and importer details
Net quantity
Batch number
Manufacturing and expiry date
Directions for use and warnings
Since post-import relabeling is not permitted, importers must finalize labels before shipment.
Non-Animal Testing Declaration
Furthermore, India prohibits the import of cosmetics tested on animals. Therefore, manufacturers and importers must submit a declaration confirming compliance with the animal testing ban.
Heavy Metals and Restricted Substance Declarations
Manufacturers must also declare compliance with limits prescribed for:
Heavy metals such as Lead, Mercury, and Arsenic
Hexachlorophene
Approved color additives
These declarations support CDSCO’s ingredient safety evaluation.
Additional Cosmetic Import License Documents (Case-Specific)
Depending on the product category and formulation, CDSCO may request additional documents such as:
Certificate of Analysis (COA)
Package inserts or leaflets
Product photographs
Safety references for borderline cosmetic products
Therefore, importers should remain prepared for product-specific documentation requirements.
Common Mistakes in Cosmetic Import License Documents
Despite clear regulations, many applications face avoidable delays. For example:
Inconsistent manufacturer details across documents
Incorrect INCI ingredient terminology
Missing apostille on Power of Attorney
Non-compliant label artwork
FSC not covering all product variants
Fortunately, most of these issues can be prevented through expert document review before submission.
How Regacats Solutions Helps with Cosmetic Import License Documents
At Regacats Solutions, we support importers throughout the cosmetic import licensing process. Specifically, we assist with:
Manufacturer coordination and document verification
Ingredient and labeling compliance checks
Preparation and filing of COS-1 applications
CDSCO query handling and follow-ups
As a result, our clients achieve faster approvals with reduced compliance risk.
Importers seeking expert assistance may also explore CDSCO cosmetic import license consulting services in India for professional support.
Conclusion
In conclusion, preparing accurate documents for cosmetic import license forms the foundation of successful cosmetic import compliance in India. While the regulations appear straightforward, execution quality determines approval success.
By organizing documents correctly and aligning them with CDSCO expectations, importers can avoid delays, reduce regulatory risk, and enter the Indian cosmetic market confidently.