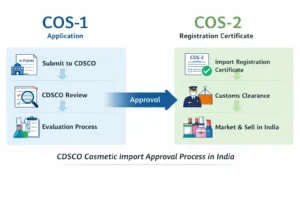
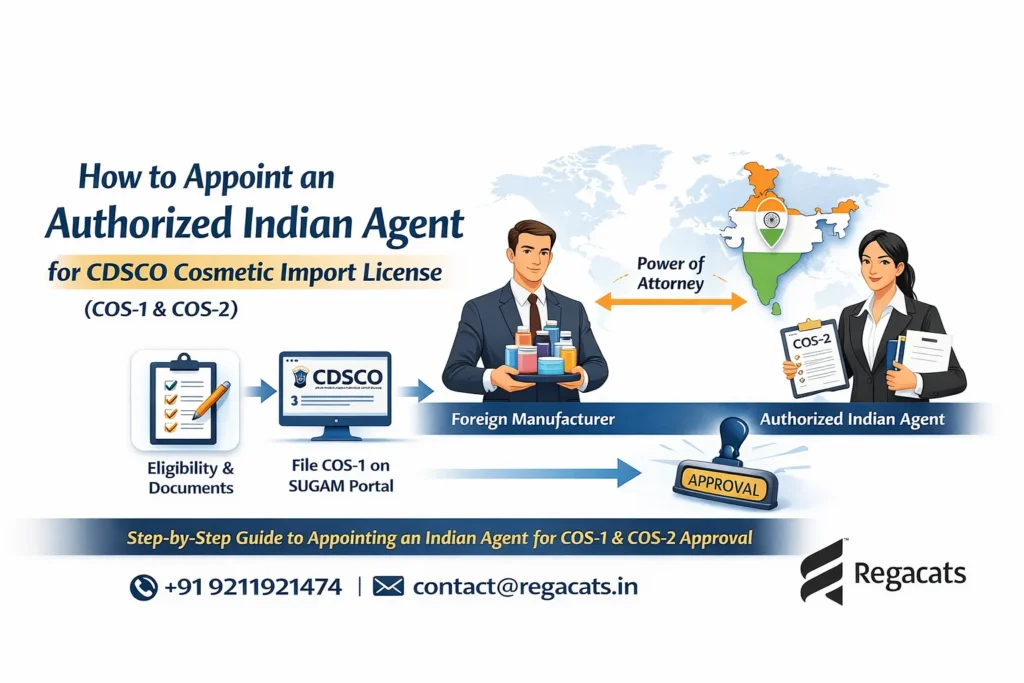
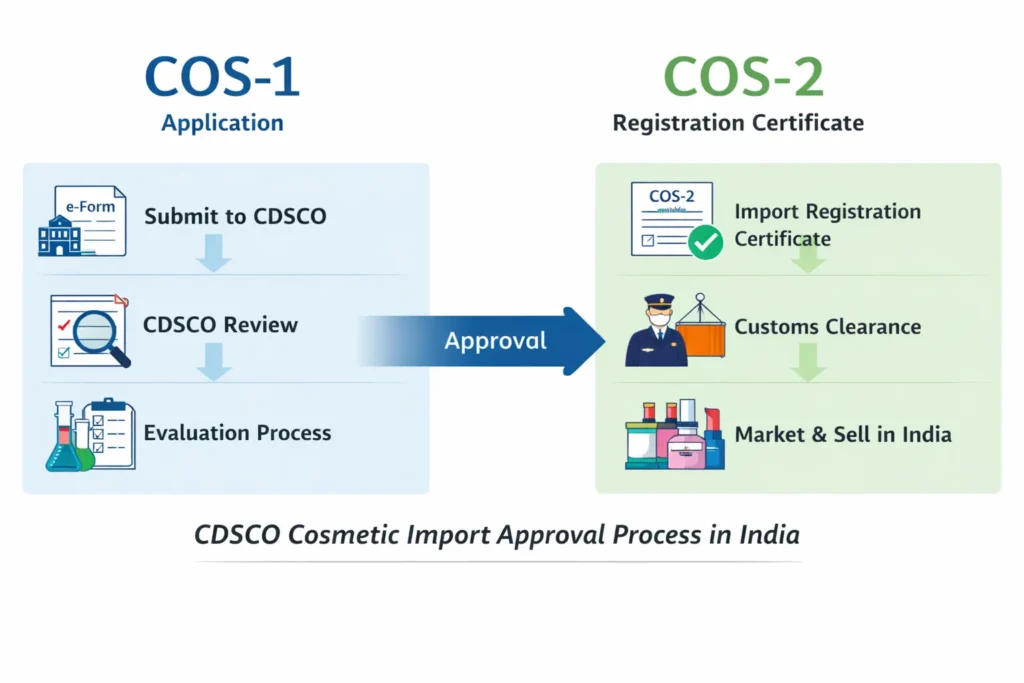
To import Korean cosmetics to India, an importer must obtain CDSCO cosmetic import registration before shipment. The process includes appointing an authorized Indian importer, submitting documents on the CDSCO SUGAM portal, paying fees, and receiving approval prior to customs clearance.
However, importing Korean cosmetics into India is not a simple trading activity. Instead, Indian law strictly regulates the process under the Drugs and Cosmetics Act, 1940, whilethe Central Drugs Standard Control Organization (CDSCO).
Based on years of experience handling cosmetic import registrations, this guide explains how to import Korean cosmetics into India step by step, covering licenses, documentation, timelines, government fees, and common compliance risks.
Table of Contents
ToggleHow to Import Korean Cosmetics into India (Quick Steps)
To begin with, importers must follow these regulatory steps:
Firstly, appoint an authorized Indian importer or agent
Next, obtain a Power of Attorney from the Korean manufacturer
Then, prepare complete product and manufacturer documentation
After that, apply online through the CDSCO SUGAM portal
Subsequently, pay the government registration fees
If required, respond to CDSCO regulatory queries
Once approved, receive CDSCO cosmetic import registration
Finally, import products through Indian customs
Understanding Korean Cosmetics Under Indian Regulations
As per Indian law, cosmetics include products intended to be applied to the human body for cleansing, beautifying, promoting attractiveness, or altering appearance.
Therefore, Korean cosmetics falling under this definition include:
Skincare products such as creams, lotions, serums, and masks
Hair care products like shampoos and conditioners
Makeup and other beauty products
Importantly, Importers must register all Korean cosmetics with CDSCO before importing them into India. Moreover, this applies whether products are sold online or offline.
Why Import Korean Cosmetics to India?
From a market and compliance perspective, Korean cosmetics offer strong potential.
Firstly, Indian consumers actively prefer K-beauty products.
Secondly, Korean brands are known for innovation.
Moreover, premium positioning supports better margins.
Additionally, e-commerce growth has expanded reach.
Finally, India’s ban on animal testing aligns with Korean standards.
Consequently, compliant importers benefit from a scalable opportunity.
Who Is Eligible to Import Korean Cosmetics into India?
From a regulatory perspective, the CDSCO allows the following entities to apply for cosmetic import registration:
An Indian importer or distributor
An authorized Indian agent appointed by the Korean manufacturer
An Indian subsidiary of the foreign manufacturer
In addition, the applicant must have:
A legally registered business entity in India
IEC (Import Export Code)
GST registration
Before shipping Korean beauty products to India, certain mandatory registrations must be completed. Otherwise, customs authorities may detain or reject the shipment.
1. CDSCO Cosmetic Import Registration
This is the core regulatory approval. Without it, cosmetics cannot be imported, stocked, sold, or marketed in India.
2. Import Export Code (IEC)
Issued by DGFT, IEC is mandatory for customs clearance.
3. GST Registration
Required for taxation and invoicing.
Step-by-Step Process to Import Korean Cosmetics into India
Step 1: Appointment of Authorized Indian Agent
The Korean manufacturer must issue a Power of Attorney authorizing an Indian importer or agent to act on its behalf.
Step 2: Documentation Preparation
This stage determines approval speed. Documents must be accurate, consistent, and compliant with Indian norms.
Step 3: Online Application on CDSCO Portal
The cosmetic import registration application is submitted online via the CDSCO SUGAM portal.
Step 4: Government Fee Payment
Fees are paid online and depend on:
- Number of Product Categories
- Number of Manufacturing site
Number of variants
Step 5: Regulatory Review by CDSCO
CDSCO reviews ingredients, labels, and manufacturer credentials. CDSCO often raises queries when applicants submit inconsistent documentation.
Step 6: Import and Customs Clearance
Once approved, the products can be legally imported and cleared through Indian customs.
Documents Required to Import Korean Cosmetics into India
Based on regulatory experience, the following documents are critical:
Power of Attorney from manufacturer
Free Sale Certificate issued in Korea
Manufacturing license
Complete ingredient list with composition
Product specifications and safety data
Label artwork as per Indian rules
Covering letter and application forms
As a result, errors in ingredients or labeling remain the most common reasons for delay.
Labeling Requirements to Import Korean Cosmetics in India
Every imported cosmetic must display:
Product name and category
Manufacturer’s name and address
Importer’s name and address
Batch number
Manufacturing and expiry date
Net quantity
“Imported by” declaration
Importantly, Importers must ensure label compliance before shipping products to India. Otherwise, custom clearance issues arise.
Government Fees for Importing Korean Cosmetics into India
CDSCO government fees depend on:
Number of product categories
- Number of Manufacturing sites
Variants under each category
Since, fees are non-refundable, making correct filing essential.
Timeline for Importing Korean Cosmetics into India
From experience, the realistic timeline is:
Approval generally takes 2–3 months. However, delays may occur.
Additional time if regulatory queries are raised
With expert handling, Regacats Solutions typically completes approvals in around 40 days.
Common Compliance Challenges Faced by Importers for Importing Korean Cosmetics Into India
Ingredient mismatches with Indian standards
Incorrect or incomplete labeling
Poorly drafted Power of Attorney
Inconsistent manufacturer details
Fortunately, most rejections are avoidable with expert compliance review.
Customs Duty & Import Charges for Korean Cosmetics in India (2026 Updated)
Customs duty varies depending on category, packaging, and HS code. On average, total landed cost including freight ranges between 40%–65% of product cost.
Skincare HS Code – 3304
Haircare HS Code – 3305
Perfumes HS Code – 3303
Therefore, cost calculation is essential to maintain profitability.
Where to Source Genuine Korean Cosmetic Brands for Import in India?
You can source products directly from Korean manufacturers or verified wholesale suppliers. Popular sourcing hubs include:
Myeongdong
Namdaemun Cosmetics Market
Wholesale platforms such as Qoo10, Alibaba, and StyleKorea
Before importing, always request MSDS, ingredient list, and export authorization.
How Regacats Solutions Facilitates the Korean Cosmetics import into India
This guide is prepared by experienced professionals in CDSCO regulatory consulting.
Regacats Solutions provides:
End-to-end CDSCO cosmetic import registration
Documentation and labeling compliance
Regulatory liaison and query handling
Ongoing compliance support
As a result, clients achieve faster approvals and long-term compliance.
Conclusion
In conclusion, importing Korean cosmetics into India is a lucrative opportunity. However, success depends on regulatory compliance, accurate documentation, and CDSCO approval. Therefore, with the right guidance, importers can confidently enter the Indian market and scale sustainably.
About Regacats Solutions
Regacats Solutions is an India-based regulatory consulting firm specializing in CDSCO and statutory compliance for regulated industries. With extensive experience in cosmetic import licensing, medical device registration, and FSSAI regulatory approvals, we assist Indian importers and international manufacturers in navigating complex Indian regulations. Our expertise covers CDSCO cosmetic import licenses (COS-1 & COS-2), medical device import registration, Legal Metrology compliance, and EPR authorization. Regacats Solutions focuses on India-specific regulatory frameworks, ensuring accurate documentation, faster approvals, and long-term compliance for businesses entering or expanding in the Indian market.