Regulatory Support for Non-specified, Novel Food or Ingredients (NSF Registration)
Food businesses introducing non-specified food or ingredient, novel food or ingredient, new ingredients or products not standardized under existing FSSAI regulations must obtain approval under the Food Safety and Standards (Approval for Non-Specified Food and Food Ingredients) Regulations, 2017 before manufacturing, importing, or marketing such ingredients or products in India.
Regacats Solutions provides specialized Non-Specified Food and Ingredient Approval services in India, supporting food, nutraceutical, and dietary supplement businesses with scientific dossier preparation, regulatory submissions, and authority coordination. With over 8 years of focused experience in Indian food regulations, we help Indian and international companies navigate one of the most complex approval pathways under FSSAI.
Our non-specified food approval support is part of our broader FSSAI regulatory consulting services in India, helping businesses ensure complete compliance.
What is Non-specified food or Ingredient:
Non-specified food or ingredient means any food other than proprietary food or food ingredients, including additives, processing aids and enzymes for which the Act does not specify standards for such foods or ingredients.
What is Novel food or food ingredient:
A novel food or food ingredient refers to a product that does not have an established history of human consumption. It may also include foods that contain ingredients, or are derived from sources, that have not traditionally been consumed by humans. In addition, foods or ingredients produced using new or innovative technologies fall under this category, especially where such processes cause a significant change in the food’s composition, structure, or particle size. These changes may impact the nutritional value, metabolism, or the level of undesirable substances present in the food.
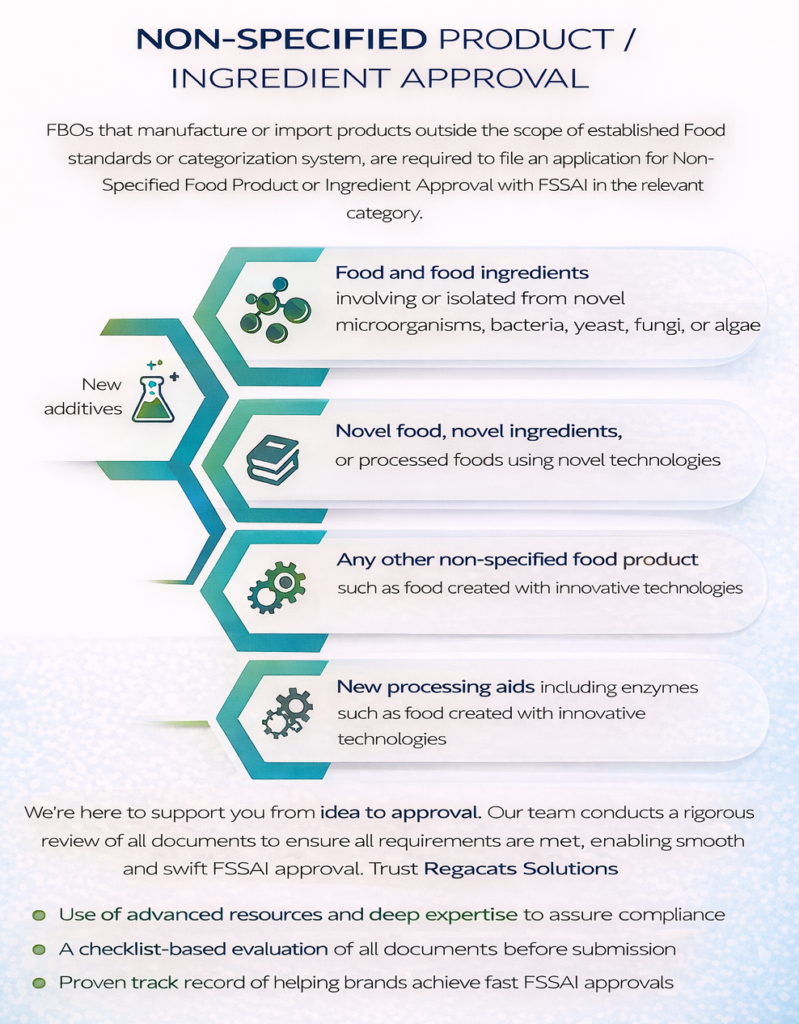
What Is Non-Specified Food or Ingredient Approval Under FSSAI?
Non-Specified Food or Ingredient approval refers to approval of any food product or ingredient that:
Is not covered under existing FSSAI food standards, and
Does not fall under any standardized category prescribed in FSSAI regulations.
Such products require FSSAI to grant prior approval before marketing in India.
Food business operators must obtain non-specified food approval in addition to a Central FSSAI license.
Operating or importing non-specified food without approval may result in:
Rejection at customs
Product seizure or recall
Regulatory notices and penalties
Market access restrictions
Who Requires Non-Specified Food / Ingredient Approval?
Non-Specified Food approval is required for businesses involved in:
Novel food products
New food ingredients or sources
Food products or ingredients prepared with novel technology
Nutraceuticals with non-specified or novel ingredients
Dietary supplements with innovative formulations
This requirement applies to both Indian manufacturers and foreign food businesses seeking to enter the Indian market.
Examples of Non-Specified Foods & Ingredients
The following products commonly fall under non-specified approval:
Novel plant extracts or bioactive compounds
New vitamins, minerals, or delivery forms
Food, Nutraceutical, Dietary Supplement products formulated with novel technology
Ingredients not listed under FSSAI regulation & schedules
Products with ingredients not approved in India
FSSAI assesses each product on a case-by-case basis.
Regulatory Framework Governing Non-Specified Food Approval
The following Act & regulations govern non-specified food and ingredient approvals:
Food Safety and Standards Act, 2006
Food Safety and Standards (Approval for Non-Specified Food and Food Ingredients) Regulations, 2017
Relevant advisories, orders, and scientific committee guidelines issued by FSSAI
FSSAI grants approvals only after conducting scientific safety evaluations
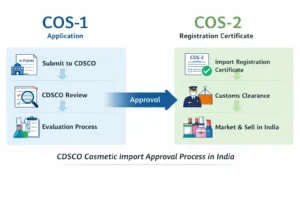
Step-by-Step Process for Non-Specified Food & Ingredient Approval
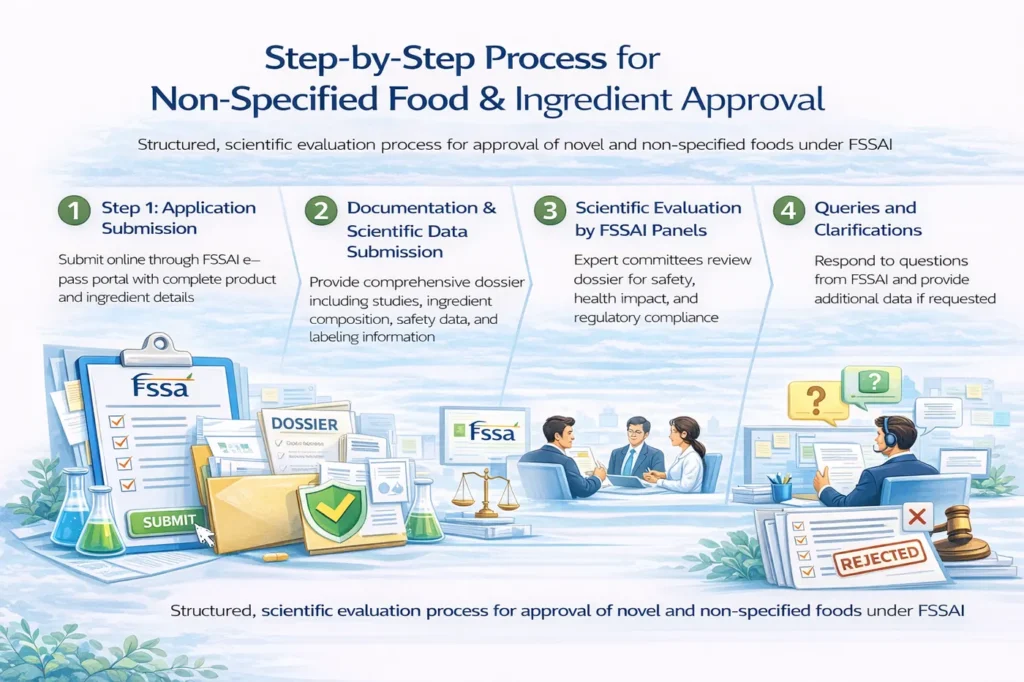
The approval of non-specified food and food ingredients under FSSAI follows a structured, scientific evaluation process to ensure safety and regulatory compliance before market entry.
Step 1: Application Submission
The application is submitted online through the FSSAI e-pass portal. The applicant is required to provide complete and accurate product and ingredient details, including intended use and category classification.
Step 2: Documentation & Scientific Data Submission
Comprehensive documentation is submitted to support the safety and regulatory acceptance of the product or ingredient. This typically includes:
Detailed ingredient composition with scientific justification
Toxicological and safety studies, where applicable
Labeling information, including nutrition facts, allergen declarations, and proposed claims
Source and nature of the ingredient (plant, animal, synthetic, or biotechnology-derived)
Manufacturing process details and country of origin, particularly for imported products
Step 3: Scientific Evaluation by FSSAI Panels
The submitted dossier is reviewed by FSSAI-designated Scientific Panels and Expert Committees, which assess the safety, health impact, usage levels, and compliance with Indian dietary and food safety norms.
Step 4: Queries and Clarifications
During the evaluation process, FSSAI may raise technical queries or request additional data, studies, or clarifications. Timely and accurate responses are critical to avoid delays.
Step 5: Approval or Rejection Decision
Based on the scientific assessment:
Approval permits the product or ingredient to be legally manufactured, imported, and marketed in India, subject to specified conditions.
Rejection may occur if safety data is insufficient or if the ingredient is determined to pose potential health risks.
Timelines & Government Fees
Indicative Timelines: Approval timelines vary depending on product complexity and scientific evaluation requirements, typically ranging from 6 to 8 months.
Government Fees: The applicable FSSAI approval fee is INR 50,000 + 18% GST, subject to change as per regulatory updates.
Our Non-Specified Food & Ingredient Approval Services
Regacats Solutions provides end-to-end regulatory support throughout the approval lifecycle.
Regulatory Classification & Feasibility Assessment
We evaluate whether your product qualifies as non-specified food, proprietary food, nutraceutical, or another regulated category, helping avoid misclassification.
Ingredient & Safety Data Review
Our experts assess ingredient safety, global regulatory status, toxicology data, and history of use to determine approval viability.
Scientific Dossier Preparation
We prepare comprehensive dossiers including:
Product and ingredient specifications
Manufacturing process details
Safety and toxicological studies
Global regulatory approvals (where applicable)
Proposed usage levels and justification
Application Submission & Authority Coordination
We manage submission through the FSSAI approval mechanism and coordinate with authorities to address queries, clarifications, and scientific panel observations.
Post-Approval Compliance Support
We assist with labeling alignment, license mapping, and ongoing compliance after approval is granted.
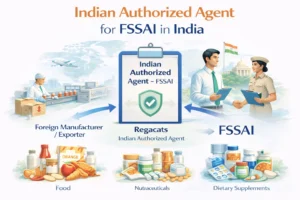
Non-Specified Food Approval for Importers & International Companies
Foreign manufacturers and global brands importing food products into India must ensure that all ingredients comply with Indian regulations. Where ingredients are non-specified, prior FSSAI approval is mandatory.
Our services for international clients include:
Ingredient gap analysis against Indian standards
Scientific dossier preparation for imported products
Central FSSAI license alignment
Indian Authorized Agent (IAA) support (if required)
Coordination with customs and port authorities
Documents Required to Submit Non-Specified Food & Ingredient Application
Product type and ingredient complexity determine the documentation requirements.
Detailed product-related information
Source of food ingredients
Manufacturing process with process flow chart
FSSAI license number (if available)
Functional use details of the product
Intended use information
Worldwide regulatory status of the product of atleast two countries
Certificate of Analysis (CoA) from an NABL accredited laboratory
Safety data
Copy of the agreement defining the relationship between the applicant, manufacturer, and other involved parties
Supporting claim substantiation through clinical trials or scientific journals
Safety assessment for sensitive groups such as pregnant women, lactating mothers, children, or other special populations
Pruduct label as per applicable FSSAI Regulations
Declaration to conduct and submit post-marketing surveillance data
Any additional documents specific to the product category and general regulatory requirements
Our regulatory team provides a customized checklist after the initial assessment.
Challenges in Non-Specified Food & Ingredient Approval
While Non-Specified Food and Ingredient approval is critical for market access, the regulatory process presents several challenges for food business operators.
1. Complex Documentation Requirements
The approval process demands extensive scientific documentation, including safety assessments, toxicological studies, and compliant labeling information. Preparing these documents in line with FSSAI expectations can be technically demanding and resource-intensive.
2. Scientific Evaluation and Regulatory Queries
Applications undergo detailed scientific review by FSSAI-appointed panels. During this stage, additional data or clarifications may be requested, which can extend approval timelines if not addressed accurately.
3. Regulatory Challenges for Imported Products
Imported food products and ingredients are subject to dual regulatory scrutiny—compliance with both the country of origin standards and Indian food safety regulations. Any gaps may result in delays or rejection at the approval or import stage.
4. Limited Awareness Among Food Businesses
Many food business operators are unaware of which products or ingredients fall under the Non-Specified Food category, leading to misclassification, incorrect filings, or inadvertent non-compliance.
Role of Expert Regulatory Consultants like Regacats Solutions in Non-Specified Food or Ingredient Approval
Engaging experienced regulatory and advisory consultants helps businesses navigate the complexity of the non-specified food approval process with greater clarity and confidence.
1. Regulatory Strategy & Product Classification
Consultants assess product composition and intended use to determine whether Non-Specified Food approval is required and identify the most appropriate regulatory pathway.
2. Scientific Documentation & Dossier Preparation
Professional support ensures the preparation of robust scientific justifications, safety data, and regulatory dossiers that align with FSSAI evaluation criteria.
3. Efficient Authority Coordination
Experienced consultants manage regulatory communications and respond to scientific queries accurately, reducing unnecessary delays and repeated submissions.
4. Risk Reduction & Compliance Assurance
By addressing regulatory expectations upfront, consultants help minimize the risk of rejection due to incomplete, inconsistent, or inadequate data.
5. Cross-Category Compliance Support
Regulatory experts provide integrated compliance guidance across food, nutraceutical, and dietary supplement categories, ensuring consistency throughout the product lifecycle.
Why Choose Regacats Solutions for Non-Specified Food Approval?
Regacats Solutions has established itself as a reliable partner for food business operators, brand owners, and manufacturers across India. With strong expertise in the FSSAI and CDSCO regulatory frameworks, the team helps turn regulatory compliance from a challenge into a driver of business growth.
8+ years of experience in Indian food regulations
Specialized expertise in novel foods and non-specified ingredients
Scientific, regulator-aligned dossier preparation
PAN India and international client support
Transparent communication and compliance-focused approach
Frequently Asked Questions – Non-Specified Food Approval
What happens if a product is sold without non-specified food approval?
Such products may face regulatory action, including rejection, recall, or penalties.
How long does the approval process take?
Timelines vary depending on product complexity and scientific evaluation requirements.
Is non-specified food approval mandatory for imports?
Yes. Imported products with non-standard ingredients require approval before import.
Can one approval cover multiple products?
Approvals are product-specific and ingredient-specific.
Does approval replace FSSAI licensing?
No. Approval is in addition to the applicable FSSAI license.