Table of Contents
ToggleRegulatory Support for Food, Nutraceutical & Dietary Supplement Claims
FSSAI Health Claim Approval plays a critical role in how food, nutraceutical, and dietary supplement products are marketed in India. However, regulators treat these claims as one of the most strictly controlled aspects of food compliance.
Any statement that suggests health benefits, disease risk reduction, enhanced bodily function, or ingredient efficacy must comply with the requirements of the Food Safety and Standards Authority of India (FSSAI).
Regacats Solutions provides end-to-end FSSAI Health Claim Approval services in India, supporting both Indian and international food business operators with scientific substantiation, regulatory submissions, and authority coordination. With over 8 years of focused experience in Indian food regulations, we help brands launch compliant products while minimizing regulatory risk.
FSSAI Health Claim Approval overview
The Food Safety and Standards Authority of India (FSSAI) acts as the statutory authority that regulates food-related claims in India under the Food Safety and Standards Act, 2006. Food businesses must obtain health claim approval when a claim:
Is not listed under Schedule III of the Food Safety and Standards (Advertising & Claims) Regulations, 2018
Relates to disease risk reduction, enhanced function, or product-led benefits
Goes beyond standard nutrient function claims
Through this approval process, FSSAI ensures that health claims remain truthful, non-misleading, scientifically substantiated, and relevant to Indian consumers.
Who Can Apply for FSSAI Health Claim Approval?
FSSAI health claim approval applies to a wide range of food business operators. For example, eligible applicants include:
Food manufacturers
Nutraceutical and dietary supplement companies
Importers of finished products
Marketers and brand owners
Re-labelers and private-label businesses
Moreover, both Indian companies and foreign manufacturers intending to market products in India must comply with this regulatory requirement.
Types of Claims Covered Under FSSAI Health Claim Approval
Food businesses may require health claim approval for the following claim categories:
Disease risk reduction claims
Ingredient-led health benefit claims
Product-led functional claims
Enhanced physiological or metabolic function claims
Importantly, FSSAI evaluates each claim independently based on scientific evidence, safety considerations, and regulatory relevance.
Regulatory Framework Governing Health Claims
FSSAI evaluates health claim approvals under the following regulatory framework:
Food Safety and Standards Act, 2006
Food Safety and Standards (Advertising and Claims) Regulations, 2018
FSSAI Nutraceutical Regulations
Scientific advisories and Expert Committee guidelines issued by FSSAI
As a result, approval decisions rely heavily on scientific evidence, cause-and-effect validation, and risk assessment.
Step-by-Step Process for FSSAI Health Claim Approval
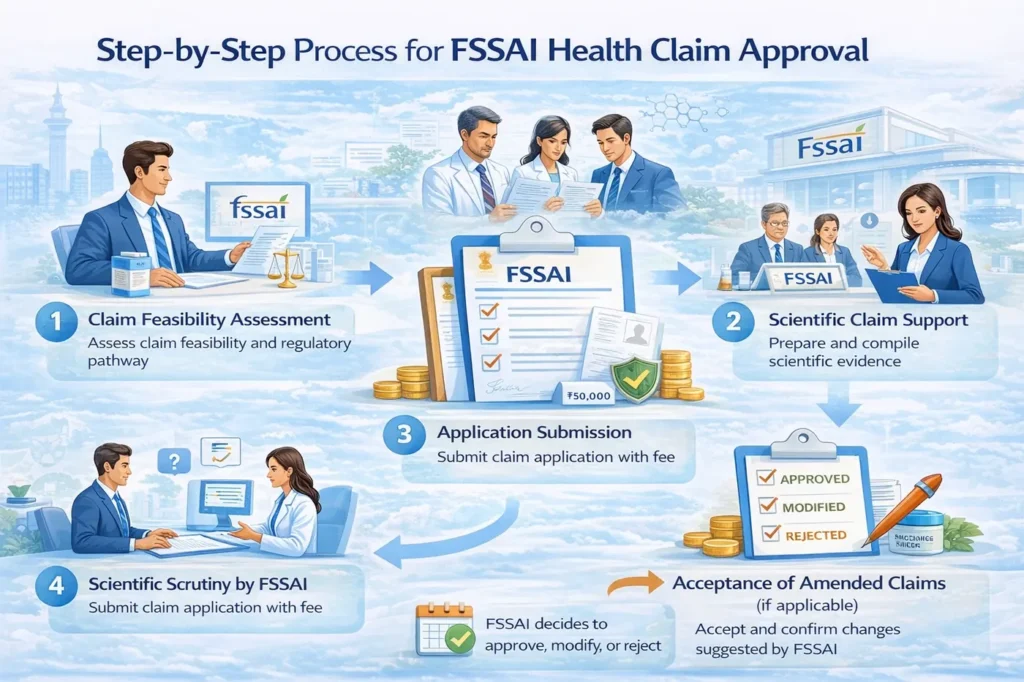
Step 1: Claim Feasibility Assessment
First, we assess whether the proposed claim is permitted, restricted, or requires prior approval under FSSAI regulations.
Step 2: Scientific Claim Support Dossier (CSD) Preparation
Next, our team prepares a detailed scientific dossier, which includes:
Published in-vitro, in-vivo, and human studies
Cause-and-effect relationship evidence
Ingredient mechanism of action
Usage levels and target population
Step 3: Application Preparation & Submission
After that, applicants prepare the application for Approval of Claims on Food Products in the prescribed format and submit it to FSSAI along with the applicable government fee of INR 50,000 + 18% GST.
Step 4: Scientific Scrutiny by FSSAI
Subsequently, FSSAI Expert Committees review the claim and supporting scientific evidence in detail.
Step 5: Regulatory Queries & Responses
During the review, FSSAI may raise deficiencies or seek clarifications. Applicants must respond within stipulated timelines to avoid delays or rejection.
Step 6: Approval, Amendment, or Rejection
Based on the evaluation, FSSAI may:
Approve the claim
Suggest claim modification
Reject the claim if evidence remains insufficient
Step 7: Acceptance of Amended Claims (if applicable)
If FSSAI suggests modifications, applicants must formally accept and communicate the revised claim for reconsideration.
Timeline & Government Fees for FSSAI Health Claim Approval in India
Indicative Timeline: Approximately 180 days, depending on claim complexity and scientific review
Government Fee: INR 50,000 + 18% GST per application
Maximum: Three claim statements per application
Notably, approved claims do not carry a fixed validity period, provided regulations remain unchanged.
Key Documents Required for FSSAI Health Claim Approval
Common documentation includes:
Claim statement with justification
Scientific substantiation (in-vitro, in-vivo, and human studies)
Cause-and-effect studies for disease risk reduction claims
Validated analytical methods
Ingredient safety and interaction data
Product composition and category details
Copy of FSSAI license
Global regulatory approvals, where applicable
IPR details, if any
After initial evaluation, we share a customized document checklist.
Businesses must also ensure they hold a valid FSSAI license before using approved health claims on product labels.
Challenges in FSSAI Health Claim Approval
Food businesses often face several challenges, including:
Inadequate or poorly structured scientific evidence
Marketing-driven claim language that conflicts with regulations
Differences between global approvals and Indian requirements
Inconsistencies across labels, advertisements, and digital platforms
Without expert guidance, these challenges frequently result in delays or regulatory rejection.
How Regacats Solutions Supports FSSAI Health Claim Approval
Regulatory Strategy & Claim Mapping
We identify the correct approval pathway and help businesses avoid unnecessary regulatory risk.
Scientific Dossier Development
Our experts prepare regulator-aligned scientific justifications that meet FSSAI expectations.
Authority Coordination
We manage communication with FSSAI and respond to Expert Committee queries accurately and on time.
Risk Mitigation
We help brands prevent penalties, product recalls, and post-market enforcement actions.
Import & Global Brand Support
Additionally, we assist foreign manufacturers in aligning global claims with Indian regulations. Imported products carrying health claims may also require assistance for import of food, supplement or nutraceutical products to ensure smooth customs clearance and regulatory compliance.
Why Choose Regacats Solutions?
8+ years of experience in Indian food regulations
Deep expertise in health claims and scientific substantiation
PAN India and international client coverage
Transparent, compliance-first approach
Trusted regulatory partner for food and nutraceutical brands
Frequently Asked Questions – FSSAI Health Claim Approval
Is health claim approval mandatory for all claims?
No. Only claims beyond standard permitted statements require approval.
Can one approval cover multiple products?
No. Approvals remain claim-specific and product-specific.
Can approved claims be used in advertising?
Yes. However, brands must maintain consistency with approved wording.
Do imported products require claim approval?
Yes. Imported products must comply with Indian claim regulations.
Can rejected claims be resubmitted?
Yes. Applicants may resubmit claims with revised scientific justification.