Foreign Food Manufacturing Facility Registration in India
Table of Contents
ToggleFSSAI Registration for Foreign Food Manufacturers Exporting to India
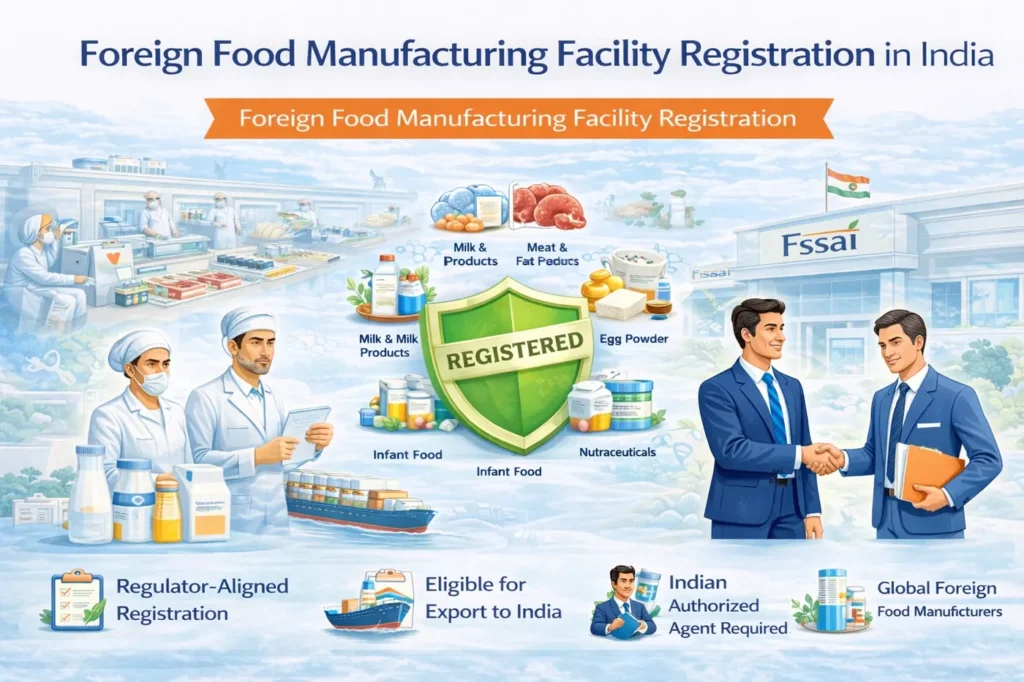
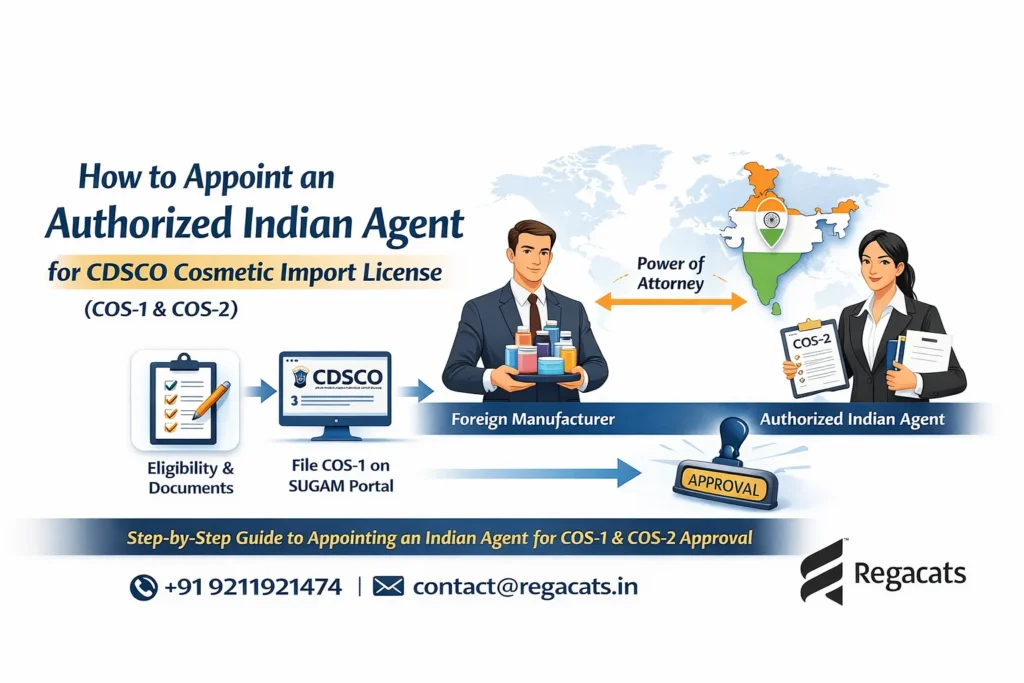
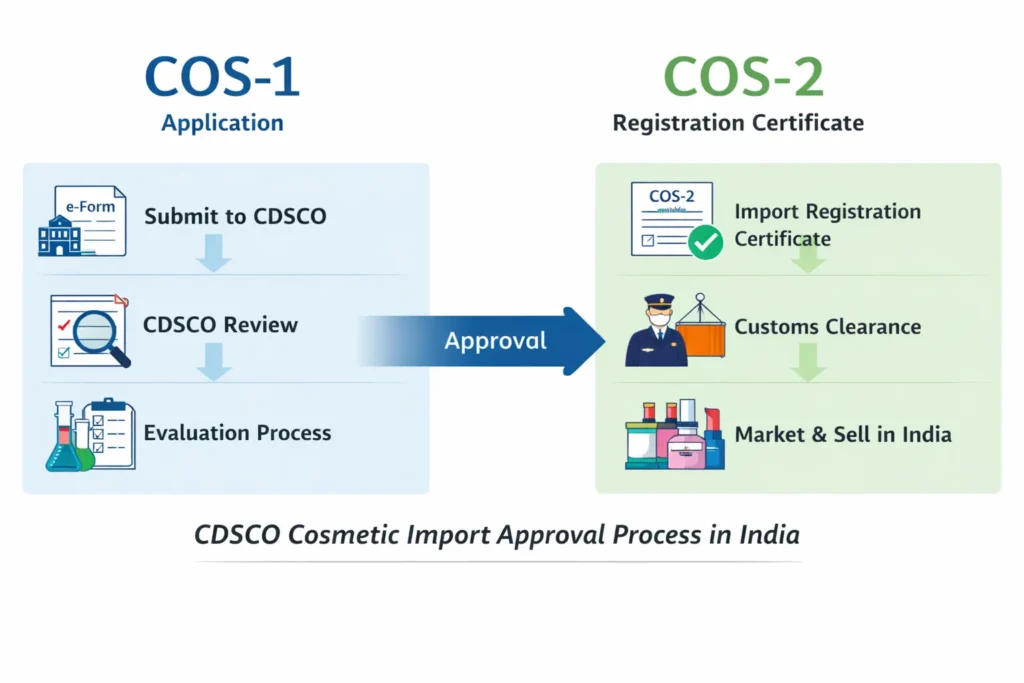
Foreign Food Manufacturing Facility Registration is mandatory for overseas food manufacturing facilities exporting specified food products to India. India follows a strict preventive food safety framework; therefore, foreign food manufacturers must comply with FSSAI requirements before exporting food products to the Indian market. Regacats Solutions supports global food manufacturers with structured, regulator-aligned foreign food facility registration in India.
What Is Foreign Food Manufacturing Facility Registration (ReFOM)?
Registration of Foreign Food Manufacturing Facility, commonly referred to as ReFOM, is an approval mechanism under FSSAI that requires certain overseas food manufacturing facilities to register before exporting food products to India.
Through this process, FSSAI evaluates:
Regulatory oversight in the exporting country
Authorization status of the manufacturing facility
Nature and category of food products proposed for export
Compliance with Indian food safety standards
Once registered, the foreign facility becomes eligible to export approved food products to India, subject to continued compliance.
Why ReFOM Registration Is Mandatory for Foreign Food Manufacturers
India follows a risk-based and preventive food safety approach. Consequently, FSSAI mandates prior registration of foreign food manufacturing facilities for selected food categories.
Without ReFOM registration:
Food consignments may be rejected at Indian ports
Import clearance may be delayed or denied
Importers may face regulatory enforcement
Market access may be restricted
Therefore, ReFOM registration establishes regulatory confidence and significantly reduces import-related risks.
Who Is Required to Apply for Foreign Food Manufacturing Facility Registration?
ReFOM registration applies to foreign food manufacturing facilities that intend to export identified or high-risk food categories to India.
Typically, this includes:
Overseas food manufacturers without a physical establishment in India
Facilities exporting food products notified by FSSAI for mandatory registration
Manufacturers supplying regulated food categories to Indian importers
Applications is mandatory to submit by the competent authority of exporting country.
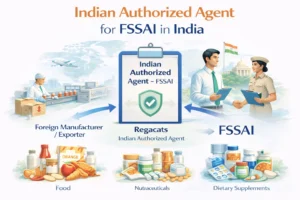
Foreign manufacturers also required to appoint an Indian Authorized Agent for FSSAI to manage regulatory coordination in India.
Food Categories Require Registration on ReFoM Portal

FSSAI mandates Foreign Food Facility Registration (ReFOM) for overseas food manufacturing facilities exporting specific food categories to India. Therefore, foreign facilities falling under the following categories must obtain registration prior to export:
Mandatory Food Categories Covered Under Foreign Food Manufacturing Facility Registration
Foreign food manufacturing facilities exporting the following categories are required to register with FSSAI:
Milk and Milk Products
This category includes milk, dairy products, processed milk foods, and dairy derivatives.Meat and Meat Products
This includes meat, poultry, fish, seafood, and all processed products derived from animal sources.Egg Powder
Facilities manufacturing egg powder and egg-based powdered ingredients must comply with ReFOM requirements.Infant Food
Manufacturers producing infant formula, infant nutrition products, and related foods require mandatory registration.Nutraceuticals
Foreign facilities manufacturing nutraceutical products intended for export to India must also register under ReFOM.
Why FSSAI Focuses on These Categories
These food categories involve higher public health and food safety risks. Therefore, FSSAI applies stricter upstream controls to ensure compliance before products enter the Indian supply chain.
As a result, mandatory facility registration helps:
Strengthen food safety oversight
Improve traceability of imported food products
Reduce import rejections and regulatory action
Protect consumer health in India
Regulatory Framework Governing ReFOM Registration
Foreign food manufacturing facility registration operates under:
Food Safety and Standards Act, 2006
Relevant FSSAI regulations, orders, and advisories
Official ReFOM application formats and procedures
FSSAI retains authority to review applications, request clarifications, conduct inspections, and grant or reject registration.
Step-by-Step Process for Foreign Food Manufacturing Facility Registration
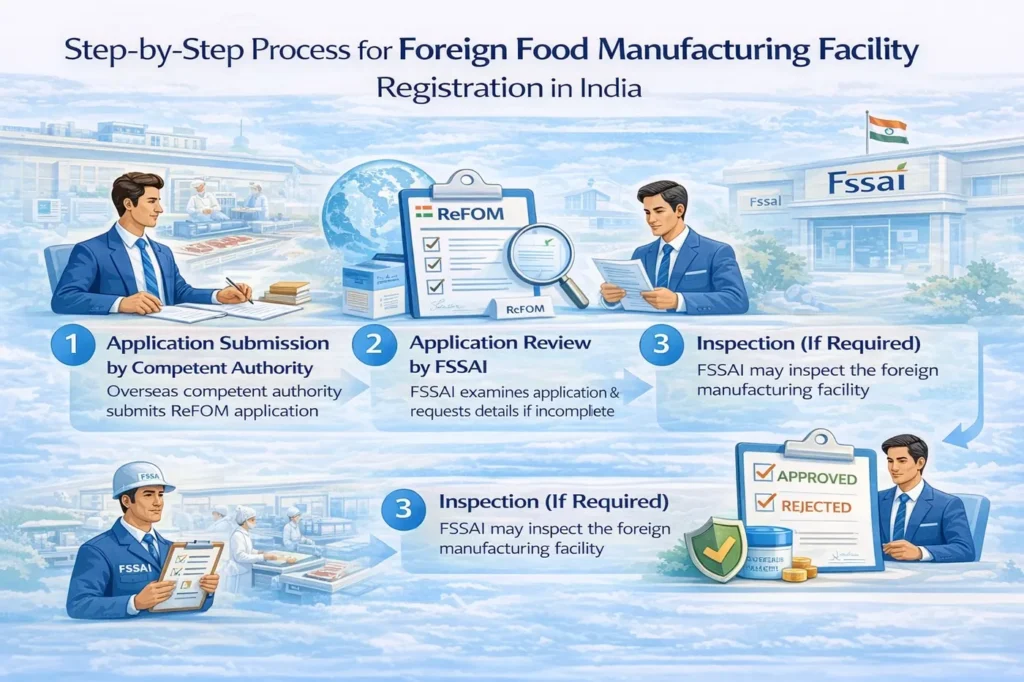
Step 1: Application Submission
Foreign food manufacturing facilities submit the ReFOM application by competent authority of exporting country.
Step 2: Application Review
FSSAI reviews the submitted application and supporting information. If the application is incomplete, the authority requests additional details.
Step 3: Inspection (If Required)
FSSAI may inspect the foreign manufacturing facility based on risk assessment. The applicant bears inspection-related costs, if applicable.
Step 4: Approval or Rejection
Based on compliance assessment, FSSAI may:
Grant registration, or
Reject the application with reasons
Facilities may reapply after implementing corrective measures.
Validity of Foreign Food Manufacturing Facility Registration
Once granted, ReFOM registration remains valid for two (2) years from the date of issuance, subject to ongoing compliance.
Fees for ReFOM Registration
Currently, there is no fee for ReFOM registration. However, inspection costs, where applicable, are payable by the foreign manufacturer.
Documents Required for Foreign Food Manufacturing Facility Registration
Foreign food manufacturing facilities must submit:
1. Country Details
Name of the exporting country
2. Competent Authority in Exporting Country
Name of competent authority
Address and official contact details
3. Manufacturing Facility Details
Name of the food manufacturing facility
Complete manufacturing site address
Official contact details
4. Approval or License Issued by Competent Authority
Valid approval or license number
5. Product Details for Export to India
Name of food product(s)
Harmonized System (HS) Code for each product
Common Challenges in ReFOM Registration
Foreign food manufacturers often face challenges such as:
Incomplete or inconsistent documentation
Delays due to inspection scheduling
Misalignment between exporting country approvals and Indian requirements
Uncertainty regarding category coverage
Early regulatory assessment significantly reduces these risks.
How Regacats Solutions Supports Foreign Food Manufacturing Facility Registration
Regacats Solutions provides structured, regulator-aligned support throughout the ReFOM lifecycle, including:
Applicability assessment
Documentation gap analysis
Application preparation and coordination
Inspection readiness guidance
Authority communication support
As a result, foreign manufacturers achieve smoother registration and predictable compliance outcomes.
This service complements our broader FSSAI regulatory services in India for global food businesses.
Why Choose Regacats Solutions?
8+ years of experience in Indian food regulations
Strong expertise in FSSAI compliance for global manufacturers
Practical understanding of export-to-India regulatory risks
Transparent, compliance-first approach
Dedicated support for foreign manufacturers and international brands
Partner with Regacats Solutions for ReFOM Compliance
Foreign food manufacturers planning to export to India should initiate ReFOM registration early to avoid regulatory delays and supply disruptions.
Regacats Solutions supports global food manufacturers with compliant, regulator-aligned Foreign Food Manufacturing Facility Registration in India, enabling sustainable access to the Indian market.
Frequently Asked Questions – Foreign Food Manufacturing Facility Registration
Is ReFOM registration mandatory for all food exports to India?
No. ReFOM applies only to notified food categories.
Can a foreign manufacturer apply without an Indian representative?
Yes. Applications may be submitted directly or through an authorized representative.
Does FSSAI always inspect foreign facilities?
No. Inspections depend on risk assessment.
What happens if registration is rejected?
Facilities may reapply after corrective action.
How long does the registration process take?
Timelines vary based on documentation quality and inspection needs.