Indian Authorized Agent for Drugs
Table of Contents
ToggleRegulatory Representation for Global Pharmaceutical Manufacturers
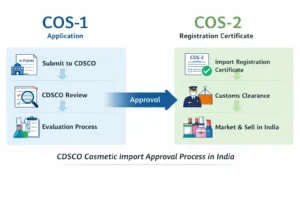
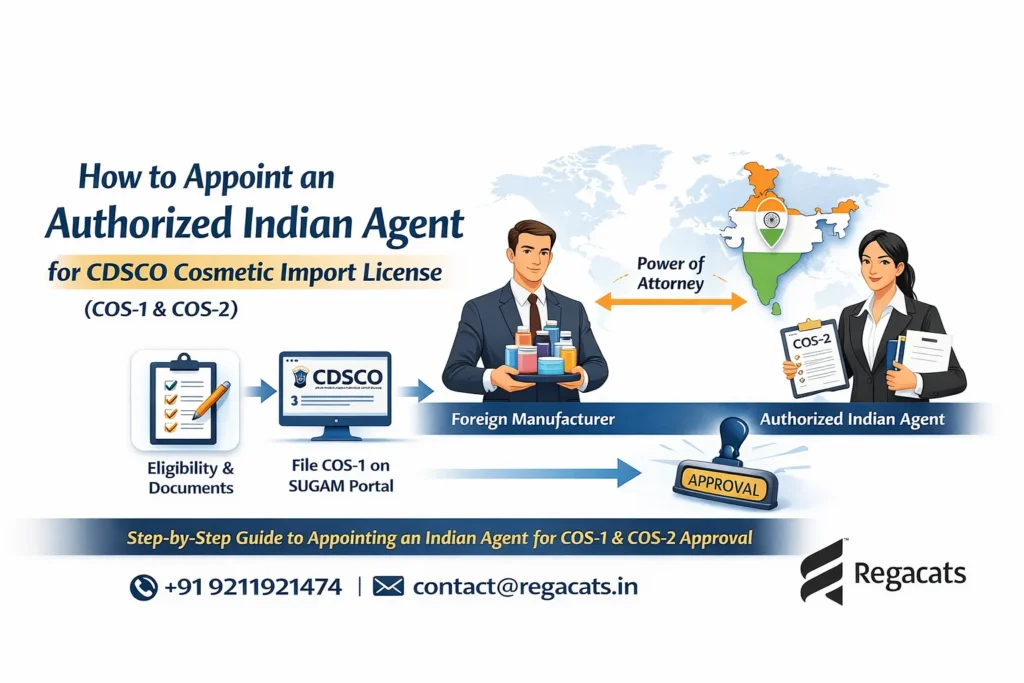
Indian Authorized Agent for Drugs services are mandatory for foreign pharmaceutical manufacturers entering the Indian market. India is one of the world’s largest pharmaceutical markets; however, drug imports and commercialization operate under a strict regulatory framework. Therefore, overseas manufacturers must comply with Indian drug regulations before importing, distributing, or marketing pharmaceutical products in India. Regacats Solutions provides Indian Authorized Agent services for drugs, supporting global pharmaceutical manufacturers with regulatory representation, compliance management, and long-term market access in India.
What Is an Indian Authorized Agent for Drugs?
An Indian Authorized Agent for drugs is a legally established Indian entity appointed by a foreign pharmaceutical manufacturer to act as its official regulatory representative in India.
The authorized agent:
Represents the foreign manufacturer before Indian drug authorities
Submits applications for drug registration and import permissions
Coordinates responses to regulatory queries and inspections
Manages post-approval compliance and vigilance obligations
As a result, the Indian Authorized Agent becomes the single point of regulatory accountability for the foreign manufacturer in India.
This service forms part of our broader Indian Authorized Agent services in India, supporting foreign manufacturers across regulated product categories.
Why Indian Authorized Agent Services Are Mandatory for Drugs in India
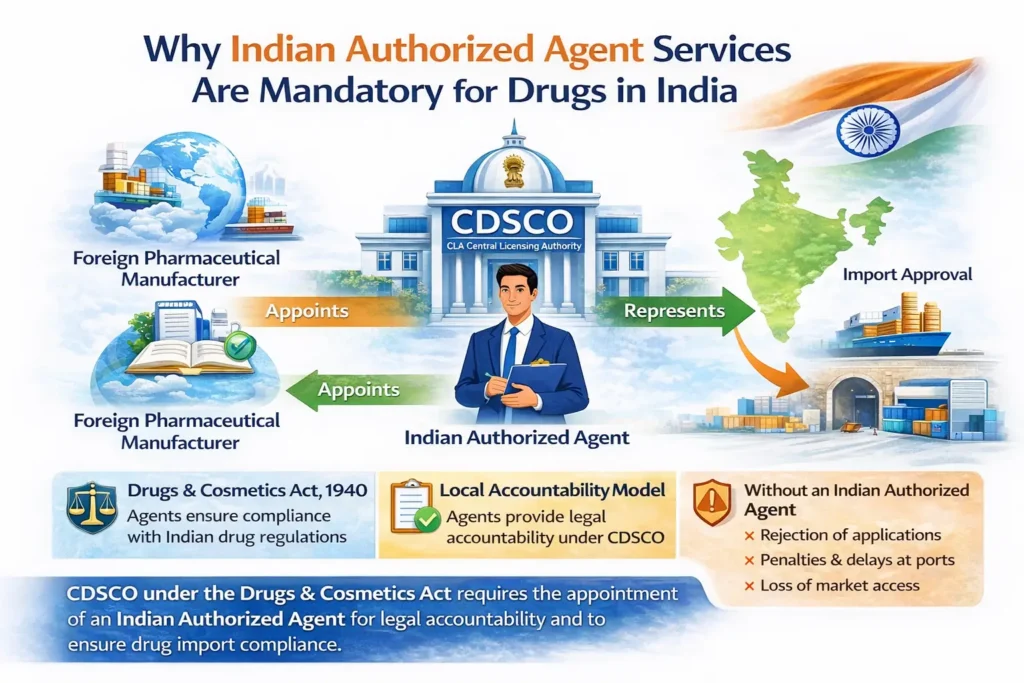
India follows a local accountability model under the Drugs and Cosmetics Act, 1940 and the Drugs and Cosmetics Rules, 1945. Consequently, foreign drug manufacturers cannot directly interact with Indian regulators without appointing an authorized representative.
Drug regulation and import oversight in India are administered by the Central Drugs Standard Control Organisation (CDSCO) through the Central Licensing Authority (CLA).
Without an Indian Authorized Agent, foreign manufacturers may face:
Rejection of drug registration applications
Import delays or refusal at Indian ports
Regulatory notices, penalties, or suspension
Loss of market access
Who Requires an Indian Authorized Agent for Drugs?
Indian Authorized Agent services apply to foreign entities involved in:
Finished pharmaceutical formulations
Bulk drugs and APIs
Biologicals and biosimilars
Vaccines and injectable products
Imported drugs for commercial sale or distribution
Moreover, this requirement applies even if the drug is already approved in other regulated markets such as the US, EU, UK, or Australia.
Key Regulatory Responsibilities of an authorized regulatory representative for pharmaceutical products
An Indian Authorized Agent for drugs performs several critical regulatory and legal responsibilities, including:
Acting as the official regulatory representative in India
Submitting drug registration applications (Form 40)
Coordinating issuance of Registration Certificate (Form 41)
Supporting procurement of import licenses (Form 10)
Managing regulatory correspondence and inspections
Supporting adverse drug reaction (ADR) reporting
Coordinating pharmacovigilance and PSUR submissions
Assisting with product recalls and corrective actions
Because these responsibilities involve legal and regulatory liability, foreign manufacturers must appoint an experienced and compliant agent.
Wholesale License Requirement for Indian Authorized Agents
Indian regulations require the authorized agent to hold a valid wholesale drug license for sale and distribution of drugs in India.
Typically, this includes:
Wholesale License in Form 20B and Form 21B
Compliance with state licensing authority requirements
Qualified personnel and compliant storage facilities
Regacats Solutions operates with a regulatory-aligned infrastructure, enabling lawful representation of foreign pharmaceutical manufacturers.
Our Indian Authorized Agent Services for Drug Compliance
Regacats Solutions provides end-to-end Indian Authorized Agent services for drugs and pharmaceutical products.
Regulatory Assessment & Product Feasibility
First, we assess product type, regulatory classification, approval status, and import feasibility under Indian drug laws.
Appointment as Indian Authorized Agent
Next, we formally act as the authorized regulatory representative for foreign manufacturers in India.
Drug Registration & Import Licensing Support
Additionally, we manage registration certificate applications, import licenses, and authority coordination through prescribed portals.
Pharmacovigilance & Post-Market Compliance
We support ADR reporting, PSUR submissions, complaint handling, and regulatory vigilance obligations.
Regulatory Queries & Recall Management
Finally, we assist with authority queries, corrective actions, and product recall coordination when required.
As a result, foreign manufacturers benefit from continuous regulatory oversight and compliance assurance.
Indian Authorized Agent vs Importer for Drugs
Foreign pharmaceutical companies often confuse the role of an Indian Authorized Agent with that of an importer. However, both roles are distinct.
Indian Authorized Agent
Handles regulatory representation
Manages registrations and compliance
Interfaces directly with CDSCO
Importer
Handles customs clearance and logistics
Manages commercial import transactions
Although one entity may perform both roles, regulatory accountability remains with the authorized agent.
Importers may also require drug import assistance to support customs clearance and regulatory documentation.
Why Global Pharmaceutical Manufacturers Choose Regacats Solutions as their Indian Agent
Global pharmaceutical manufacturers choose Regacats Solutions because we combine regulatory expertise with execution discipline.
8+ years of experience in Indian drug regulations
Expertise in import, registration, and post-market compliance
Proven support for global pharmaceutical companies
Transparent, compliance-first approach
PAN India operational capability
Single point of accountability for Indian authorities
Therefore, our clients reduce regulatory risk while accelerating compliant market entry.
Importance of Choosing the right Indian regulatory representative for drugs
An inexperienced or non-compliant authorized agent can expose foreign manufacturers to:
Registration delays or rejection
Import disruptions
Enforcement action and penalties
Reputational and commercial risk
By contrast, Regacats Solutions ensures regulatory continuity, compliance integrity, and long-term market stability.
Our Structured Approach as Indian Authorized Agent for Drugs
We follow a structured compliance model:
Regulatory assessment and product mapping
Formal appointment as Indian Authorized Agent
Registration and import license submission
Continuous authority coordination
Pharmacovigilance and post-market compliance
This structured approach ensures end-to-end regulatory coverage for pharmaceutical manufacturers.
Partner with Regacats Solutions as Your Indian Authorized Agent for Drugs
Foreign pharmaceutical manufacturers planning to enter or expand in India should appoint an experienced Indian Authorized Agent early to avoid regulatory delays and compliance risks.
Regacats Solutions supports global pharmaceutical manufacturers as a trusted Indian Authorized Agent, enabling compliant, controlled, and successful market entry into India.
Frequently Asked Questions – Indian Authorized Agent for Drugs
Is an Indian Authorized Agent mandatory for imported drugs?
Yes. Indian law requires foreign drug manufacturers to appoint an Indian Authorized Agent.
Can one authorized agent represent multiple drugs?
Yes, subject to regulatory scope and license conditions.
Does the authorized agent need a wholesale license?
Yes. A valid wholesale drug license is mandatory.
Can a foreign manufacturer change its authorized agent?
Yes, subject to regulatory procedures and authority approval.
Is pharmacovigilance the responsibility of the authorized agent?
Yes. The agent supports ADR reporting, PSURs, and recall actions.