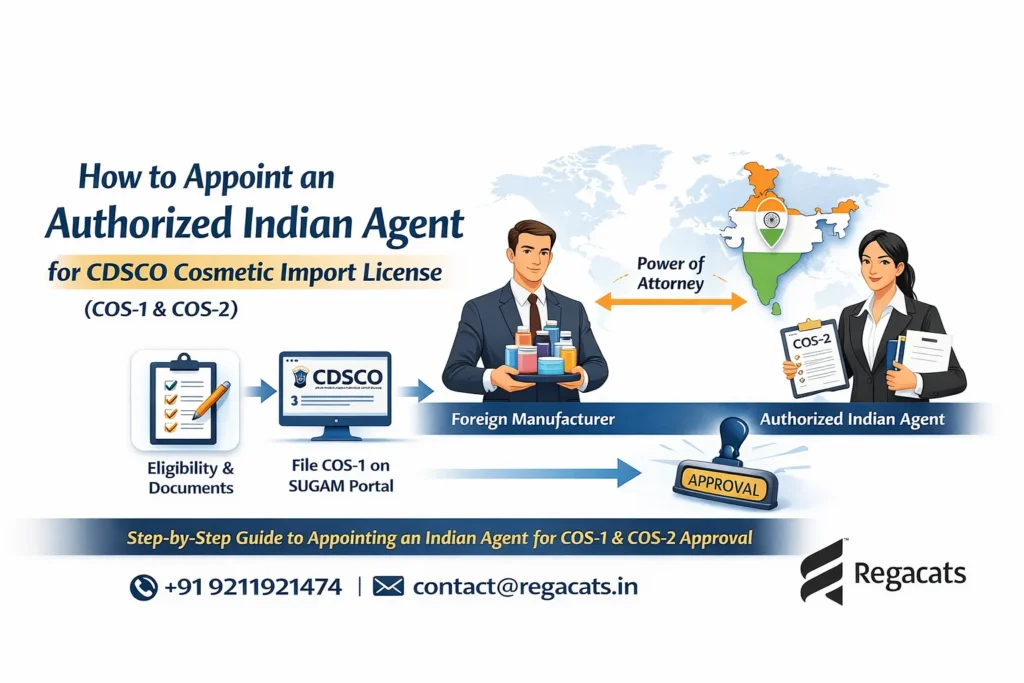
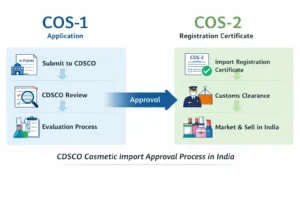
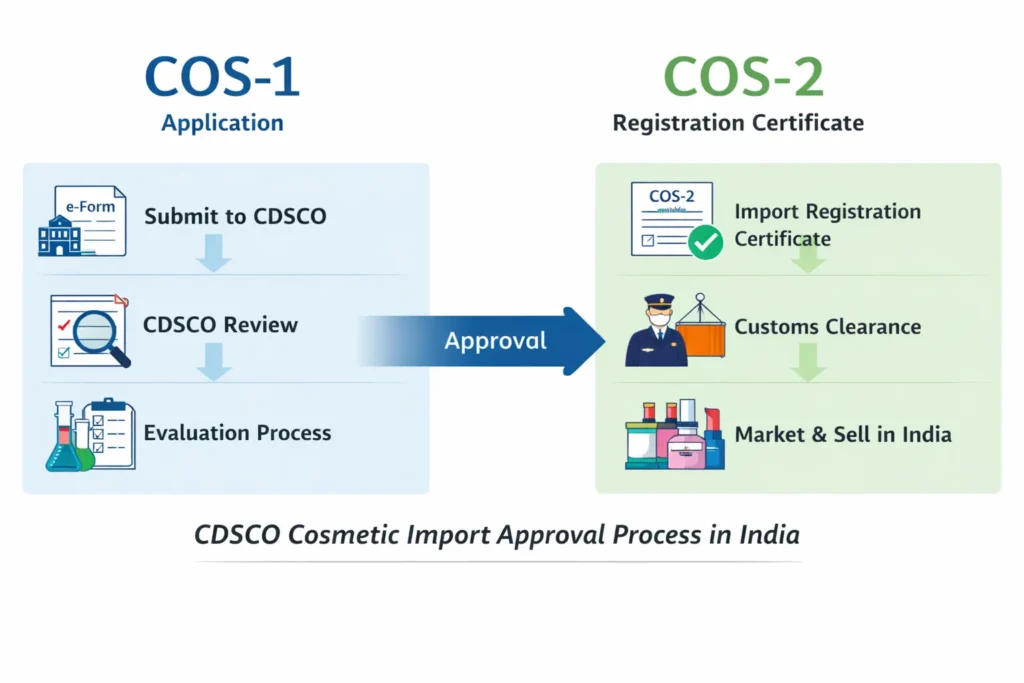
If you’re looking to appoint an Authorized Indian agent for CDSCO cosmetic Import License, understanding the regulatory framework is crucial. The authorized agent for cosmetic import or export in india plays a central role in every COS-1 and COS-2 application. First and foremost, foreign manufacturers must appoint an Indian agent because CDSCO accepts registration applications only from entities within India. Moreover, a correct appointment prevents customs delays, regulatory queries, and costly re-submissions. Therefore, this guide explains who can act as your agent, what documents you need, a step-by-step appointment process, and how Regacats Solutions supports you end-to-end.
Page Contents
ToggleWhy an Authorized Indian Agent Matters
An authorized agent acts as the legal and operational link between the foreign manufacturer and CDSCO. Consequently, the agent files the COS-1 application on the SUGAM portal, answers regulator queries, and receives the COS-2 registration certificate on behalf of the manufacturer. In addition, the agent bears responsibility for label compliance, record keeping, and post-market obligations. Thus, appointing the right agent reduces approval time and lowers regulatory risk.
Who Can Be an Indian Authorized Agent for Cosmetic Import License?
Eligible Entities to appoint as Indian Authorized Agent for Cosmetic Import License
Indian private limited companies or LLPs that hold a valid Import Export Code (IEC) and GST registration.
Indian subsidiaries of the foreign manufacturer (if legally registered in India).
Indian distributors or importers formally authorized by the manufacturer via Power of Attorney (PoA).
Who Should NOT Be an Indian Authorized Agent for Cosmetic Import License
Individuals without a registered Indian business entity.
Unofficial middlemen who cannot demonstrate IEC, GST, or a legal office address.
Choosing the wrong agent often leads to delays, regulatory non-compliance, and shipment detention.
Eligibility Requirements (Checklist)
To qualify as an authorized Indian agent, the entity must have:
A legally registered business in India (Company/LLP).
Valid Import Export Code (IEC).
GST registration.
A physical office address and local contact person.
Capability to receive legal notices/shareholder declarations if necessary.
Legal Documents Required to Appoint the Indian Authorized Agent for Cosmetic Import License
You must prepare the following key documents:
Power of Attorney (PoA) — notarized and, where required, apostilled or consularized. The PoA must explicitly authorize the Indian agent to file COS-1 and represent the foreign manufacturer before CDSCO.
Manufacturer’s Authorization / Undertaking — confirming the brand permits the agent to act on its behalf.
Company documents of the Indian agent — Incorporation Certificate, GST, IEC, and proof of office address.
Free Sale Certificate (FSC) from the country of origin for the product(s).
Product dossier — ingredient list (INCI), product specification, safety data, and label artwork.
Tip: prepare all documents in English and ensure names/addresses match across every file to avoid queries.
Step-by-Step: How to Appoint an Indian Authorized Agent for Cosmetic Import License
Step 1 — Select the Right Agent
First, shortlist agents with CDSCO experience in cosmetics. Next, verify IEC, GST, and local presence. Finally, check references and past COS-1/COS-2 approvals.
Step 2 — Draft & Execute Power of Attorney
Then, draft a clear PoA that lists:
Authorized acts (file COS-1, receive COS-2, liaise with CDSCO).
Signatory details and validity period.
After that, notarize and obtain apostille/consular legalization if CDSCO requires it for your country.
Step 3 — Compile Manufacturer & Product Documents
Meanwhile, the manufacturer compiles ingredient lists, CoA/CoC where available, label proofs, and Free Sale Certificate. Ensure all claims and ingredient names match Indian labeling rules.
Step 4 — Agent Registers on CDSCO SUGAM Portal
Next, the authorized agent registers or uses an existing account on the CDSCO SUGAM portal, completes company profile, and confirms contact details.
Step 5 — File COS-1 via SUGAM
Then, the agent uploads the completed COS-1 form, attaches PoA, FSC, product dossier, and fee challan, and submits the application.
Step 6 — Respond to CDSCO Queries
After submission, the agent monitors the application. If CDSCO raises queries, the agent coordinates prompt, precise responses with the manufacturer. Finally, when CDSCO approves, it issues the COS-2 certificate to the Indian agent.
CDSCO SUGAM Portal: Practical Notes
Use consistent file names and formats (PDF, clearly legible).
Upload a signed PoA in the required format.
Ensure the fee challan references the correct product categories and variants because fees are non-refundable.
Track the application daily; timely replies to queries shorten approval time.
Agent’s Role After COS-2 Approval
Once CDSCO issues COS-2, the agent must:
Keep registration records accessible for customs and inspections.
Ensure labeling and batch records remain compliant.
Manage renewals or endorsements for additional variants.
Coordinate with customs for clearance and document checks.
Thus, the agent plays an ongoing compliance role even after approval.
Common Risks & How to Avoid Them while appointing Indian Authorized Agent for Cosmetic Import License
Risk: Weak or invalid PoA.
Action: Use precise PoA wording and get proper notarization/legalization.Risk: Mismatched manufacturer details.
Action: Verify names, addresses, and license numbers across documents.Risk: Incorrect fee payment or category selection.
Action: Confirm product classification before paying; consult experts if unsure.Risk: Agent lacks CDSCO experience.
Action: Choose agents with a proven record of COS-1/COS-2 approvals.
How Regacats Solutions Helps Exporters & Foreign Manufacturers
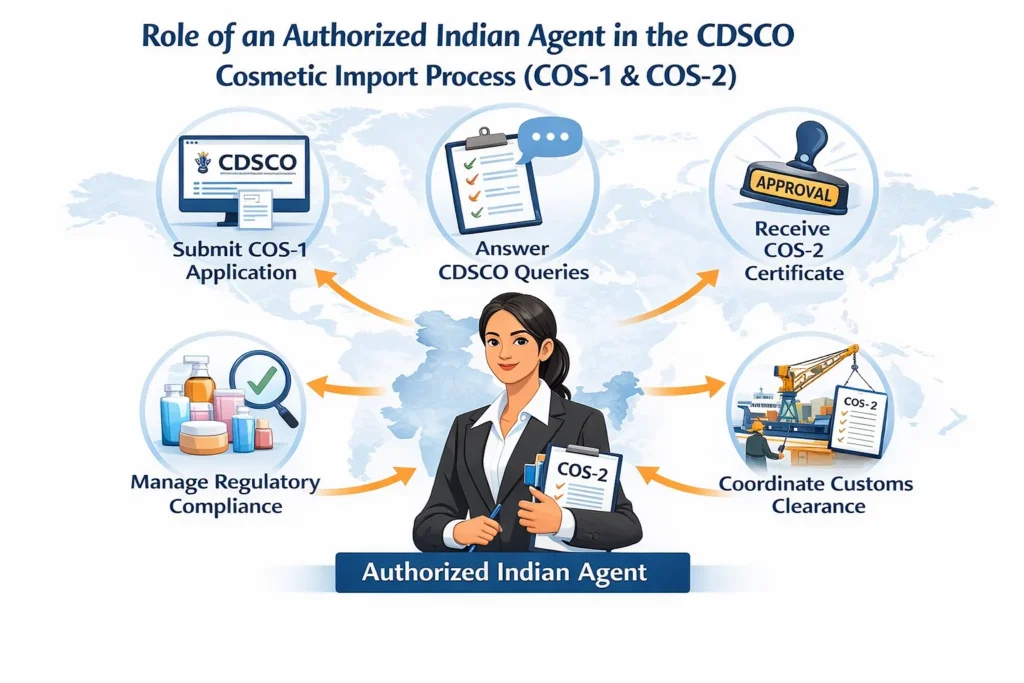
Regacats Solutions acts as a trusted authorized Indian agent and regulatory partner for foreign manufacturers and Indian importers. Specifically, we:
Draft and review Power of Attorney and manufacturer authorizations.
Prepare product dossiers, label reviews, and safety declarations.
File COS-1 on SUGAM, pay fees, and handle CDSCO queries.
Support customs clearance and post-approval compliance.
For expert support, contact us or request a free assessment through our service page: CDSCO Cosmetic Import License Consultant in India. Additionally, for a comprehensive regulatory overview, see Cos-1 & COS-2 Complete Guide
Quick Checklist: Documents Provide to Indian Authorized Agent for Cosmetic Import License from Foreign Manufacturer
Notarized & legalized Power of Attorney
Free Sale Certificate or Market Authorization
Complete ingredient list (INCI) and product specifications
Label artwork (final and inner) compliant with Indian rules
Manufacturer’s license or undertaking and CoA where applicable
Importer details (IEC, GST) if the agent will handle customs
Summary
To appoint an authorized Indian agent for CDSCO COS-1 & COS-2, the foreign manufacturer issues a notarized Power of Attorney to an Indian company with IEC and GST. The agent files COS-1 on SUGAM, responds to CDSCO queries, and receives the COS-2 registration certificate for approved products.
FAQ on Indian Authorized Agent for Cosmetics
Reviewed by Regulatory Experts at Regacats Solutions
Reviewed by Regulatory Experts at Regacats Solutions — specialists in CDSCO cosmetic import licensing, Legal Metrology compliance, and EPR authorization in India.
Conclusion & Next Steps
Appointing the right indian authorized agent for CDSCO cosmetic import license or registration reduces regulatory risk and accelerates market entry. Therefore, prepare accurate documents, verify your agent’s credentials, and stay responsive to CDSCO queries. If you prefer professional handling, Regacats Solutions can act as your authorized Indian agent and manage the entire COS-1/COS-2 process end-to-end. Contact us today to start your COS-1 application and secure COS-2 approval smoothly.
Contact:
Email: contact@regacats.in — Phone: +91 9211921474 — Get a free consultation